Abstract
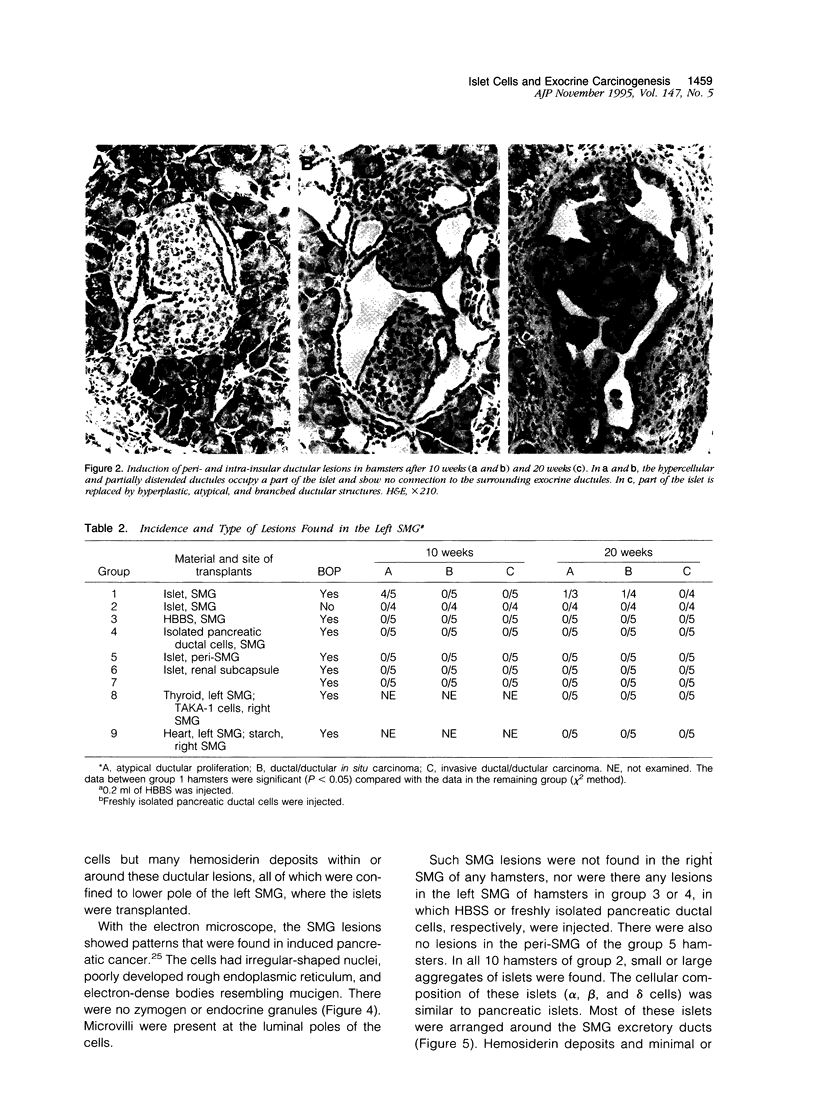
Our previous studies have suggested that the presence of intact islets is essential for the induction of pancreatic exocrine tumors in the Syrian hamster model. To validate this, we investigated the effect of the carcinogen, N-nitrosobis(2-oxo-propyl)amine (BOP) in hamsters, in which homologous isolated intact islets were transplanted into the submandibular gland (SMG). Freshly isolated pure islets from hamster donors were transplanted into the left SMG of 20 female host hamsters. Ten of these hamsters (group 1) received BOP (40 mg/kg) weekly for 3 weeks. Another 10 hamsters (group 2) were kept untreated. In groups 3 and 4 (10 hamsters each) the salt solution or isolated pancreatic ductal cells, respectively, was injected into the gland. In other groups (10 hamsters each) islets were transplanted into the peri-SMG connective tissue (group 5) or into the renal subcapsular space (group 6). Hamsters of group 1 (40 mg/kg, weekly for 3 weeks) as were group 7 hamsters, which served as BOP-treated controls. All BOP-treated hamsters developed pancreatic lesions. Similar hyperplastic and atypical ductal/ductular proliferation and in situ carcinoma were found in the SMG of many group 1 hamsters. No such lesions were found in the SMG, peri-SMG, or renal subcapsular space of the other groups. Islets appear to be involved in carcinogenicity of BOP. The mechanism is obscure.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althoff J., Pour P., Malick L., Wilson R. B. Pancreatic neoplasms induced in Syrian golden hamsters. I. Scanning electron microscopic observations. Am J Pathol. 1976 Jun;83(3):517–530. [PMC free article] [PubMed] [Google Scholar]
- Bax J., Pour P. M., Nagel D. L., Lawson T. A., Woutersen R. A., Scherer E. Long-term persistence of DNA alkylation in hamster tissues after N-nitrosobis(2-oxopropyl)amine. J Cancer Res Clin Oncol. 1990;116(2):149–155. doi: 10.1007/BF01612669. [DOI] [PubMed] [Google Scholar]
- Bell R. H., Jr, McCullough P. J., Pour P. M. Influence of diabetes on susceptibility to experimental pancreatic cancer. Am J Surg. 1988 Jan;155(1):159–164. doi: 10.1016/s0002-9610(88)80274-5. [DOI] [PubMed] [Google Scholar]
- Bell R. H., Jr, Pour P. M. Pancreatic carcinogenicity of N-nitrosobis(2-oxopropyl)-amine in diabetic and non-diabetic Chinese hamsters. Cancer Lett. 1987 Feb;34(2):221–230. doi: 10.1016/0304-3835(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Bell R. H., Jr, Sayers H. J., Pour P. M., Ray M. B., McCullough P. J. Importance of diabetes in inhibition of pancreatic cancer by streptozotocin. J Surg Res. 1989 May;46(5):515–519. doi: 10.1016/0022-4804(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Bell R. H., Jr, Strayer D. S. Streptozotocin prevents development of nitrosamine-induced pancreatic cancer in the Syrian hamster. J Surg Oncol. 1983 Dec;24(4):258–262. doi: 10.1002/jso.2930240404. [DOI] [PubMed] [Google Scholar]
- Hehmke B., Kohnert K. D., Odselius R. The use of a new dextran gradient medium for rapid isolation of functionally intact neonatal rat pancreatic islets. Diabetes Res. 1986 Jan;3(1):13–16. [PubMed] [Google Scholar]
- Ishikawa O., Wada A., Oohigashi H., Imaoka S., Iwanaga T. Relationship between goblet cells and carcinoma of the pancreas during N-nitrosobis(2-hydroxypropyl)amine-induced carcinogenesis in Syrian golden hamsters. Cancer Res. 1984 Apr;44(4):1630–1634. [PubMed] [Google Scholar]
- Pour P. M., Donnelly K., Stepan K. Modification of pancreatic carcinogenesis in the hamster model. 3. Inhibitory effect of alloxan. Am J Pathol. 1983 Mar;110(3):310–314. [PMC free article] [PubMed] [Google Scholar]
- Pour P. M. Experimental pancreatic cancer. Am J Surg Pathol. 1989;13 (Suppl 1):96–103. [PubMed] [Google Scholar]
- Pour P. M. Histogenesis of exocrine pancreatic cancer in the hamster model. Environ Health Perspect. 1984 Jun;56:229–243. doi: 10.1289/ehp.8456229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour P. M. Induction of unusual pancreatic neoplasms, with morphologic similarity to human tumors, and evidence for their ductal/ductular cell origin. Cancer. 1985 May 15;55(10):2411–2416. doi: 10.1002/1097-0142(19850515)55:10<2411::aid-cncr2820551019>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Kazakoff K., Carlson K. Inhibition of streptozotocin-induced islet cell tumors and N-nitrosobis(2-oxopropyl)amine-induced pancreatic exocrine tumors in Syrian hamsters by exogenous insulin. Cancer Res. 1990 Mar 1;50(5):1634–1639. [PubMed] [Google Scholar]
- Pour P. M., Kazakoff K., Dulaney K. A new multilabeling technique for simultaneous demonstration of different islet cells in permanent slides. Int J Pancreatol. 1993 Apr;13(2):139–142. doi: 10.1007/BF02786082. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Runge R. G., Birt D., Gingell R., Lawson T., Nagel D., Wallcave L., Salmasi S. Z. Current knowledge of pancreatic carcinogenesis in the hamster and its relevance to the human disease. Cancer. 1981 Mar 15;47(6 Suppl):1573–1589. doi: 10.1002/1097-0142(19810315)47:6+<1573::aid-cncr2820471420>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Uchida E., Burnett D. A., Steplewski Z. Blood-group antigen expression during pancreatic cancer induction in hamsters. Int J Pancreatol. 1986 Dec;1(5-6):327–340. doi: 10.1007/BF02801865. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Weide L. G., Ueno K., Corra S., Kazakoff K. Submandibular gland as a site for islet transplantation. Int J Pancreatol. 1992 Oct;12(2):187–191. doi: 10.1007/BF02924644. [DOI] [PubMed] [Google Scholar]
- Pour P. Islet cells as a component of pancreatic ductal neoplasms. I. Experimental study: ductular cells, including islet cell precursors, as primary progenitor cells of tumors. Am J Pathol. 1978 Feb;90(2):295–316. [PMC free article] [PubMed] [Google Scholar]
- Pour P., Mohr U., Cardesa A., Althoff J., Krüger F. W. Pancreatic neoplasms in an animal model: morphological, biological, and comparative studies. Cancer. 1975 Aug;36(2):379–389. doi: 10.1002/1097-0142(197508)36:2<379::aid-cncr2820360213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Moyer M. P., Cano M., Wang Q. J., Mountjoy C. P., Sanger W., Adrian T. E., Sugiura H., Katoh H., Pour P. M. Differences in molecular biological, biological and growth characteristics between the immortal and malignant hamster pancreatic cells. Carcinogenesis. 1995 Apr;16(4):931–939. doi: 10.1093/carcin/16.4.931. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Egami H., Pour P. M. Blood group antigen expression in developing pancreas and in induced pancreatic cancer cells in Syrian hamsters. Carcinogenesis. 1990 Apr;11(4):577–582. doi: 10.1093/carcin/11.4.577. [DOI] [PubMed] [Google Scholar]
- Tomioka T., Fujii H., Egami H., Takiyama Y., Pour P. M. Correlation between morphology and blood group-related antigen expression in pancreatic tumors induced in Syrian hamsters. Carcinogenesis. 1991 Mar;12(3):441–447. doi: 10.1093/carcin/12.3.441. [DOI] [PubMed] [Google Scholar]