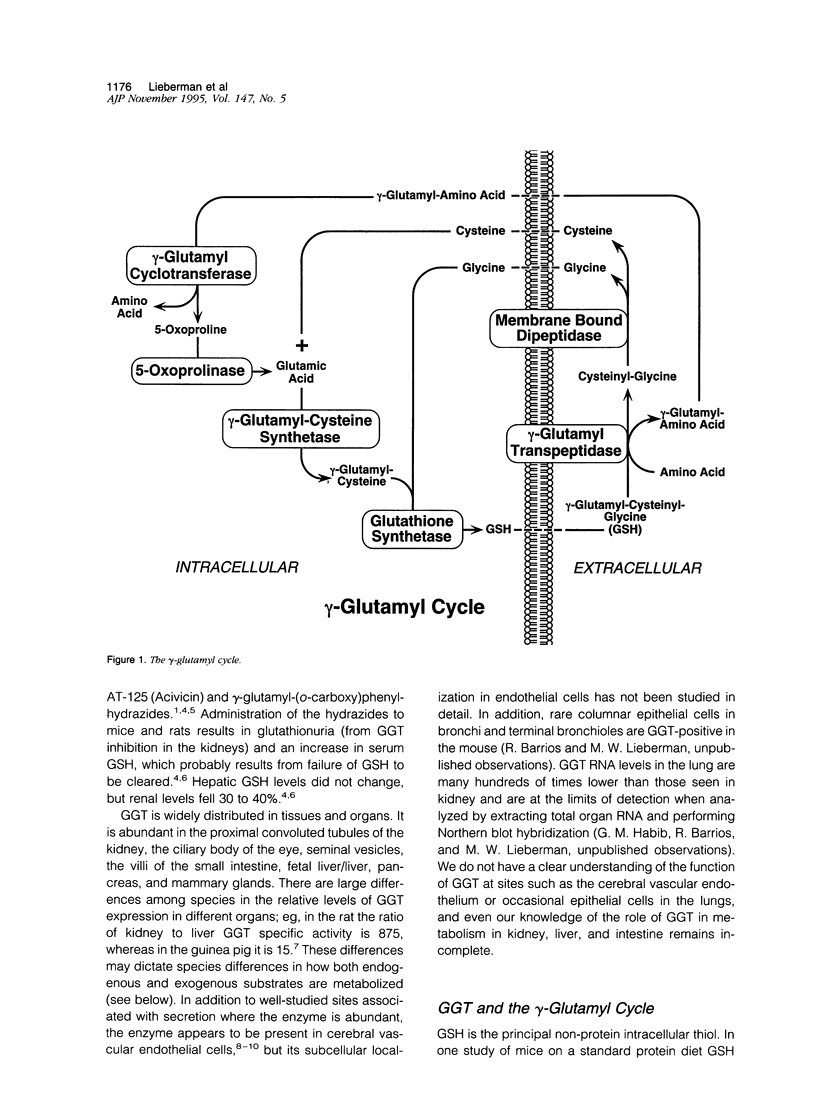
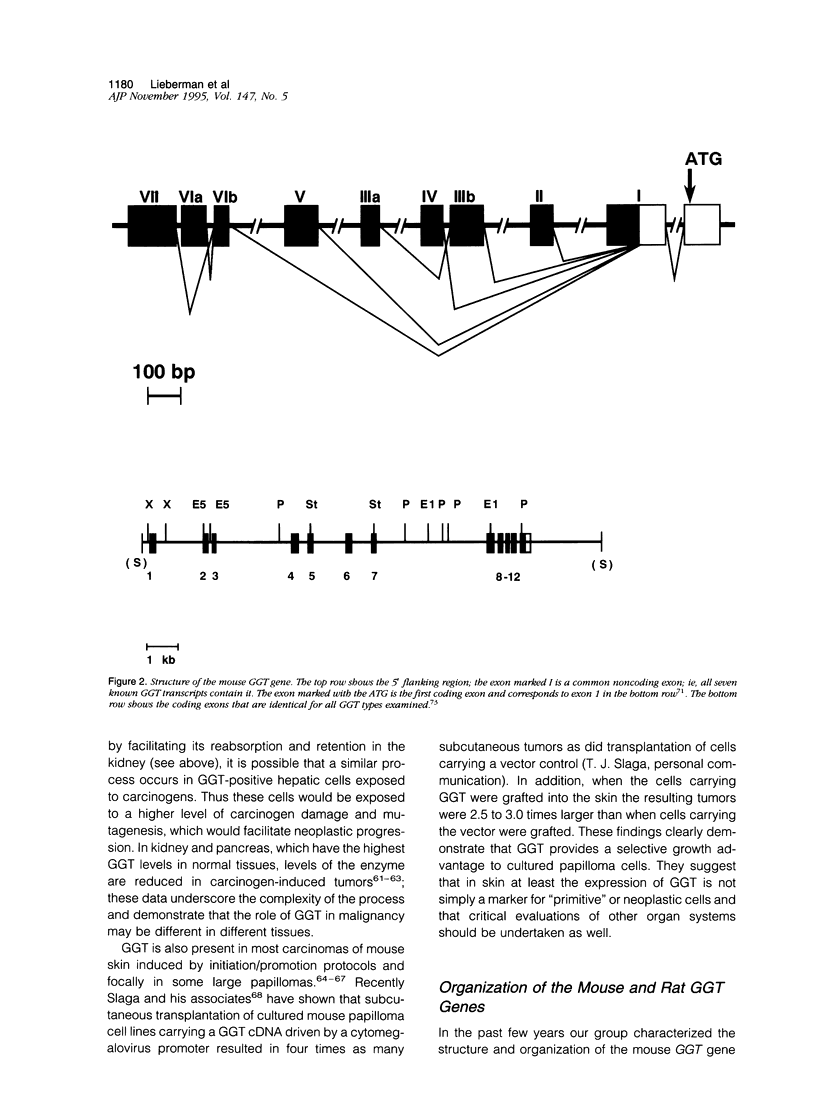
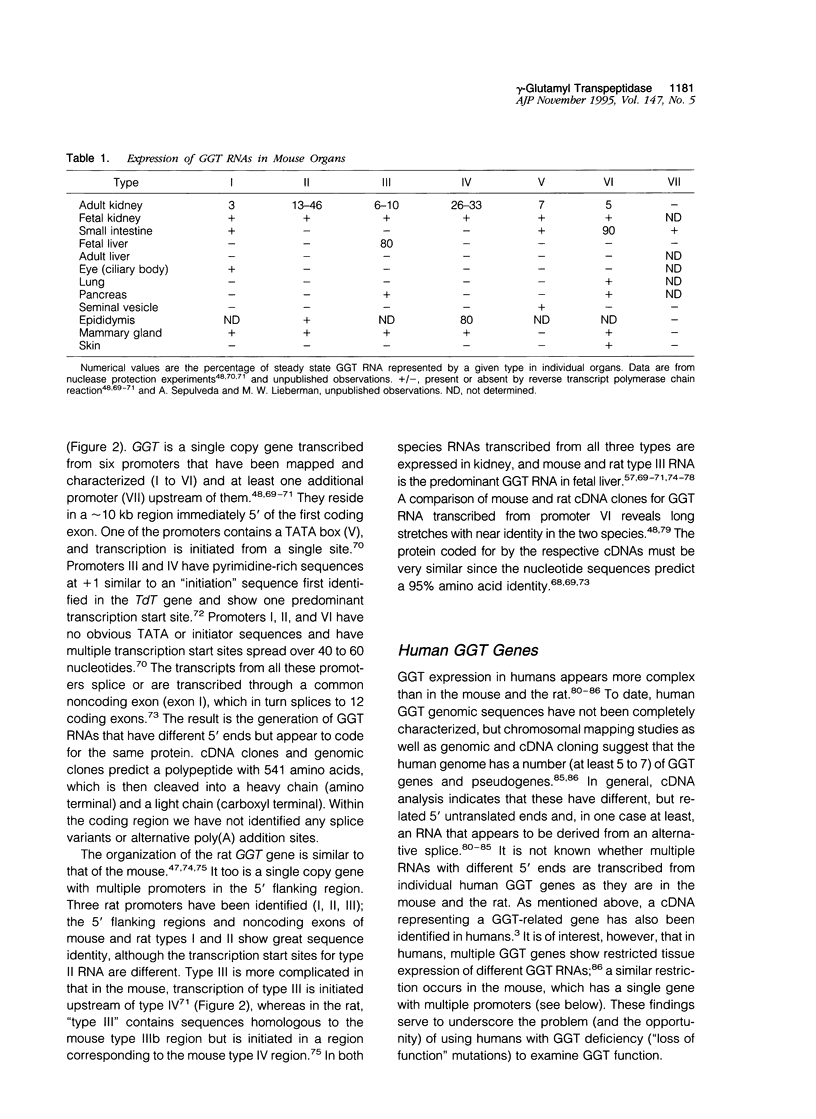
Abstract
gamma-Glutamyl transpeptidase is a key enzyme in glutathione (GSH) salvage, metabolism of endogenous mediators such as leukotrienes and prostaglandins, detoxification of xenobiotics including environmentally important compounds and carcinogens, and cellular processes dependent on the oxidation/reduction of glutathione. The enzyme is widely distributed, and these functions often occur in separate tissues and in response to different stimuli. Evidence indicates that gamma-glutamyl transpeptidase plays a direct role in some hepatic and renal responses to injury. In the mouse gamma-glutamyl transpeptidase is a single copy gene expressed from at least seven promoters, and many of the transcribed gamma-glutamyl transpeptidase RNAs are restricted in their expression. Studies that combine analyses of cellular processes with a knowledge of gene structure and expression hold promise for unravelling how these two different levels of function are integrated.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Yasutake A., Hirayama K. Influence of dietary protein levels on the fate of methylmercury and glutathione metabolism in mice. Toxicology. 1992;72(1):17–26. doi: 10.1016/0300-483x(92)90082-p. [DOI] [PubMed] [Google Scholar]
- Aldaz C. M., Conti C. J., Larcher F., Trono D., Roop D. R., Chesner J., Whitehead T., Slaga T. J. Sequential development of aneuploidy, keratin modifications, and gamma-glutamyltransferase expression in mouse skin papillomas. Cancer Res. 1988 Jun 1;48(11):3253–3257. [PubMed] [Google Scholar]
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Antoine B., Visvikis A., Thioudellet C., Rahimi-Pour A., Strazielle N., Wellman M., Siest G. Electrophoretic mobility of gamma-glutamyltransferase in rat liver subcellular fractions. Evidence for structure difference from the kidney enzyme. Biochem J. 1989 Sep 1;262(2):535–539. doi: 10.1042/bj2620535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazy M., Murphy R. C. Determination of sulfidopeptide leukotrienes in biological fluids by gas chromatography/mass spectrometry. Anal Chem. 1986 May;58(6):1098–1101. doi: 10.1021/ac00297a026. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992 Nov;263(5 Pt 1):G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Batt A. M., Siest G., Magdalou J., Galteau M. M. Enzyme induction by drugs and toxins. Clin Chim Acta. 1992 Jul 31;209(1-2):109–121. doi: 10.1016/0009-8981(92)90342-n. [DOI] [PubMed] [Google Scholar]
- Bibas M., Zampa G., Procopio A., Guaitolini R. High serum gamma-glutamyltransferase concentrations in a family. N Engl J Med. 1994 Jun 23;330(25):1832–1833. doi: 10.1056/NEJM199406233302518. [DOI] [PubMed] [Google Scholar]
- Brouillet A., Darbouy M., Okamoto T., Chobert M. N., Lahuna O., Garlatti M., Goodspeed D., Laperche Y. Functional characterization of the rat gamma-glutamyl transpeptidase promoter that is expressed and regulated in the liver and hepatoma cells. J Biol Chem. 1994 May 27;269(21):14878–14884. [PubMed] [Google Scholar]
- Carter B. Z., Habib G. M., Sepulveda A. R., Barrios R., Wan D. F., Lebovitz R. M., Lieberman M. W. Type VI RNA is the major gamma-glutamyl transpeptidase RNA in the mouse small intestine. J Biol Chem. 1994 Oct 7;269(40):24581–24585. [PubMed] [Google Scholar]
- Carter B. Z., Habib G. M., Shi Z. Z., Sepulveda A. R., Lebovitz R. M., Lieberman M. W. Rat small intestine expresses primarily type VI gamma-glutamyl transpeptidase RNA. Biochem Mol Biol Int. 1995 May;35(6):1323–1330. [PubMed] [Google Scholar]
- Caspers M. L., Diglio C. A. Expression of gamma-glutamyltranspeptidase in a transformed rat cerebral endothelial cell line. Biochim Biophys Acta. 1984 Feb 17;803(1-2):1–6. doi: 10.1016/0167-4889(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Coloma J., Pitot H. C. Characterization and sequence of a cDNA clone of gamma-glutamyltranspeptidase. Nucleic Acids Res. 1986 Feb 11;14(3):1393–1403. doi: 10.1093/nar/14.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtay C., Heisterkamp N., Siest G., Groffen J. Expression of multiple gamma-glutamyltransferase genes in man. Biochem J. 1994 Feb 1;297(Pt 3):503–508. doi: 10.1042/bj2970503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbouy M., Chobert M. N., Lahuna O., Okamoto T., Bonvalet J. P., Farman N., Laperche Y. Tissue-specific expression of multiple gamma-glutamyl transpeptidase mRNAs in rat epithelia. Am J Physiol. 1991 Dec;261(6 Pt 1):C1130–C1137. doi: 10.1152/ajpcell.1991.261.6.C1130. [DOI] [PubMed] [Google Scholar]
- De Young L. M., Richards W. L., Bonzelet W., Tsai L. L., Boutwell R. K. Localization and significance of gamma-glutamyltranspeptidase in normal and neoplastic mouse skin. Cancer Res. 1978 Nov;38(11 Pt 1):3697–3701. [PubMed] [Google Scholar]
- DeBault L. E., Cancilla P. A. gamma-Glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science. 1980 Feb 8;207(4431):653–655. doi: 10.1126/science.6101511. [DOI] [PubMed] [Google Scholar]
- Denzlinger C., Guhlmann A., Scheuber P. H., Wilker D., Hammer D. K., Keppler D. Metabolism and analysis of cysteinyl leukotrienes in the monkey. J Biol Chem. 1986 Nov 25;261(33):15601–15606. [PubMed] [Google Scholar]
- Diglio C. A., Wolfe D. E., Meyers P. Transformation of rat cerebral endothelial cells by Rous sarcoma virus. J Cell Biol. 1983 Jul;97(1):15–21. doi: 10.1083/jcb.97.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutczak W. J., Ballatori N. gamma-Glutamyltransferase-dependent biliary-hepatic recycling of methyl mercury in the guinea pig. J Pharmacol Exp Ther. 1992 Aug;262(2):619–623. [PubMed] [Google Scholar]
- Dutczak W. J., Clarkson T. W., Ballatori N. Biliary-hepatic recycling of a xenobiotic: gallbladder absorption of methyl mercury. Am J Physiol. 1991 Jun;260(6 Pt 1):G873–G880. doi: 10.1152/ajpgi.1991.260.6.G873. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Faribault G., Wiebkin P., Hamilton J. W., Longnecker D. S., Curphey T. J. gamma-Glutamyltransferase activity in atypical acinar cell nodules of rat pancreas. Toxicol Appl Pharmacol. 1987 May;88(3):338–345. doi: 10.1016/0041-008x(87)90209-2. [DOI] [PubMed] [Google Scholar]
- Fausto N. Oval cells and liver carcinogenesis: an analysis of cell lineages in hepatic tumors using oncogene transfection techniques. Prog Clin Biol Res. 1990;331:325–334. [PubMed] [Google Scholar]
- Figlewicz D. A., Delattre O., Guellaen G., Krizus A., Thomas G., Zucman J., Rouleau G. A. Mapping of human gamma-glutamyl transpeptidase genes on chromosome 22 and other human autosomes. Genomics. 1993 Aug;17(2):299–305. doi: 10.1006/geno.1993.1325. [DOI] [PubMed] [Google Scholar]
- Fryer A. A., Zhao L., Alldersea J., Pearson W. R., Strange R. C. Use of site-directed mutagenesis of allele-specific PCR primers to identify the GSTM1 A, GSTM1 B, GSTM1 A,B and GSTM1 null polymorphisms at the glutathione S-transferase, GSTM1 locus. Biochem J. 1993 Oct 1;295(Pt 1):313–315. doi: 10.1042/bj2950313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N. Enzyme patterns in human hepatocellular carcinoma. Am J Pathol. 1980 Feb;98(2):395–400. [PMC free article] [PubMed] [Google Scholar]
- Goodspeed D. C., Dunn T. J., Miller C. D., Pitot H. C. Human gamma-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene. 1989 Mar 15;76(1):1–9. doi: 10.1016/0378-1119(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Goyette M., Faris R., Braun L., Hixson D., Fausto N. Expression of hepatocyte and oval cell antigens in hepatocellular carcinomas produced by oncogene-transfected liver epithelial cells. Cancer Res. 1990 Aug 1;50(15):4809–4817. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. A., Manson M. M. Rat liver gamma glutamyl transpeptidase mRNA differs in the 5' untranslated sequence from the corresponding kidney mRNA. Cancer Lett. 1989 Jul 1;46(1):69–74. doi: 10.1016/0304-3835(89)90217-6. [DOI] [PubMed] [Google Scholar]
- Habib G. M., Carter B. Z., Sepulveda A. R., Shi Z. Z., Wan D. F., Lebovitz R. M., Lieberman M. W. Identification of a sixth promoter that directs the transcription of gamma-glutamyl transpeptidase type III RNA in mouse. J Biol Chem. 1995 Jun 9;270(23):13711–13715. doi: 10.1074/jbc.270.23.13711. [DOI] [PubMed] [Google Scholar]
- Habib G. M., Rajagopalan S., Godwin A. K., Lebovitz R. M., Lieberman M. W. The same gamma-glutamyl transpeptidase RNA species is expressed in fetal liver, hepatic carcinomas, and rasT24-transformed rat liver epithelial cells. Mol Carcinog. 1992;5(1):75–80. doi: 10.1002/mc.2940050112. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Pitot H. C. Gamma-glutamyl transpeptidase--its role in hepatocarcinogenesis. Carcinogenesis. 1985 Feb;6(2):165–172. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Rajpert-De Meyts E., Uribe L., Forman H. J., Groffen J. Identification of a human gamma-glutamyl cleaving enzyme related to, but distinct from, gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6303–6307. doi: 10.1073/pnas.88.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich S., Pitot H. C. Enzymes of glutathione metabolism as biochemical markers during hepatocarcinogenesis. Cancer Metastasis Rev. 1987;6(2):155–178. doi: 10.1007/BF00052847. [DOI] [PubMed] [Google Scholar]
- Hinchman C. A., Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J Toxicol Environ Health. 1994 Apr;41(4):387–409. doi: 10.1080/15287399409531852. [DOI] [PubMed] [Google Scholar]
- Hinchman C. A., Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990 Sep 1;40(5):1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- Humphreys W. G., Kim D. H., Cmarik J. L., Shimada T., Guengerich F. P. Comparison of the DNA-alkylating properties and mutagenic responses of a series of S-(2-haloethyl)-substituted cysteine and glutathione derivatives. Biochemistry. 1990 Nov 13;29(45):10342–10350. doi: 10.1021/bi00497a008. [DOI] [PubMed] [Google Scholar]
- Inouye M., Kajiwara Y. Strain difference of the mouse in manifestation of hydrocephalus following prenatal methylmercury exposure. Teratology. 1990 Feb;41(2):205–210. doi: 10.1002/tera.1420410212. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Müller M., Klünemann C., Schaub T., Keppler D. ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem. 1990 Nov 5;265(31):19279–19286. [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Jalanko H. Alpha-fetoprotein and gamma-glutamyltranspeptidase in mice. Effect of Raf gene. Int J Cancer. 1979 Oct 15;24(4):394–397. doi: 10.1002/ijc.2910240403. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Nelson K. G., Shah Y., Slaga T. J. Simultaneous appearance of keratin modifications and gamma-glutamyltransferase activity as indicators of tumor progression in mouse skin papillomas. J Natl Cancer Inst. 1983 Jan;70(1):161–168. [PubMed] [Google Scholar]
- Koob M., Dekant W. Bioactivation of xenobiotics by formation of toxic glutathione conjugates. Chem Biol Interact. 1991;77(2):107–136. doi: 10.1016/0009-2797(91)90068-i. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Lieberman M. W. Two genes associated with liver cancer are regulated by different mechanisms in rasT24 transformed liver epithelial cells. Oncogene. 1989 Jun;4(6):795–798. [PubMed] [Google Scholar]
- Li Y. C., Seyama T., Godwin A. K., Winokur T. S., Lebovitz R. M., Lieberman M. W. MTrasT24, a metallothionein-ras fusion gene, modulates expression in cultured rat liver cells of two genes associated with in vivo liver cancer. Proc Natl Acad Sci U S A. 1988 Jan;85(2):344–348. doi: 10.1073/pnas.85.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Koya G., Takeuchi T. Fetal Minamata disease. A neuropathological study of two cases of intrauterine intoxication by a methyl mercury compound. J Neuropathol Exp Neurol. 1965 Oct;24(4):563–574. [PubMed] [Google Scholar]
- Metafora S., Felsani A., Cotrufo R., Tajana G. F., Di Iorio G., Del Rio A., De Prisco P. P., Esposito V. Neural control of gene expression in the skeletal muscle fibre: the nature of the lesion in the muscular protein-synthesizing machinery following denervation. Proc R Soc Lond B Biol Sci. 1980 Aug 13;209(1175):239–255. doi: 10.1098/rspb.1980.0093. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. Methyl mercuric chloride toxicokinetics in mice. I: Effects of strain, sex, route of administration and dose. Pharmacol Toxicol. 1991 Mar;68(3):201–207. doi: 10.1111/j.1600-0773.1991.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Oida T., Humphreys W. G., Guengerich F. P. Preparation and characterization of oligonucleotides containing S-[2-(N7-guanyl)ethyl]glutathione. Biochemistry. 1991 Oct 29;30(43):10513–10522. doi: 10.1021/bi00107a021. [DOI] [PubMed] [Google Scholar]
- Oster T., Thioudellet C., Chevalot I., Masson C., Wellman M., Marc A., Siest G. Induction of recombinant human gamma-glutamyl transferase by sodium butyrate in transfected V79 and CHO Chinese hamster cells. Biochem Biophys Res Commun. 1993 May 28;193(1):406–412. doi: 10.1006/bbrc.1993.1638. [DOI] [PubMed] [Google Scholar]
- Ozawa N., Guengerich F. P. Evidence for formation of an S-[2-(N7-guanyl)ethyl]glutathione adduct in glutathione-mediated binding of the carcinogen 1,2-dibromoethane to DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5266–5270. doi: 10.1073/pnas.80.17.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Laneuville O., Su W. G., Corey E. J., Gurevich N., Wu P., Carlen P. L. A glutathione conjugate of hepoxilin A3: formation and action in the rat central nervous system. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3037–3041. doi: 10.1073/pnas.87.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak A., Cohen E. H., Octave J. N., Schweickhardt R., Wu S. J., Bulle F., Chikhi N., Baik J. H., Siegrist S., Guellaën G. An alternatively processed mRNA specific for gamma-glutamyl transpeptidase in human tissues. J Biol Chem. 1990 Feb 25;265(6):3256–3262. [PubMed] [Google Scholar]
- Pawlak A., Wu S. J., Bulle F., Suzuki A., Chikhi N., Ferry N., Baik J. H., Siegrist S., Guellaën G. Different gamma-glutamyl transpeptidase mRNAs are expressed in human liver and kidney. Biochem Biophys Res Commun. 1989 Oct 31;164(2):912–918. doi: 10.1016/0006-291x(89)91545-3. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Park J. H., Patel P. D., Lebovitz R. M., Lieberman M. W. Cloning and analysis of the rat gamma-glutamyltransferase gene. J Biol Chem. 1990 Jul 15;265(20):11721–11725. [PubMed] [Google Scholar]
- Rajagopalan S., Wan D. F., Habib G. M., Sepulveda A. R., McLeod M. R., Lebovitz R. M., Lieberman M. W. Six mRNAs with different 5' ends are encoded by a single gamma-glutamyltransferase gene in mouse. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6179–6183. doi: 10.1073/pnas.90.13.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpert-De Meyts E., Heisterkamp N., Groffen J. Cloning and nucleotide sequence of human gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8840–8844. doi: 10.1073/pnas.85.23.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamuro D., Yamazoe M., Matsuda Y., Kangawa K., Taniguchi N., Matsuo H., Yoshikawa H., Ogasawara N. The primary structure of human gamma-glutamyl transpeptidase. Gene. 1988 Dec 15;73(1):1–9. doi: 10.1016/0378-1119(88)90307-1. [DOI] [PubMed] [Google Scholar]
- Sepulveda A. R., Carter B. Z., Habib G. M., Lebovitz R. M., Lieberman M. W. The mouse gamma-glutamyl transpeptidase gene is transcribed from at least five separate promoters. J Biol Chem. 1994 Apr 8;269(14):10699–10705. [PubMed] [Google Scholar]
- Sinha S., Marshall C. J., Neal G. E. gamma-Glutamyltranspeptidase and the ras-induced transformation of a rat liver cell line. Cancer Res. 1986 Mar;46(3):1440–1445. [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T., Naganuma A., Imura N. Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther. 1993 Feb;264(2):776–782. [PubMed] [Google Scholar]
- Taniguchi N., Iizuka S., Zhe Z. N., House S., Yokosawa N., Ono M., Kinoshita K., Makita A., Sekiya C. Measurement of human serum immunoreactive gamma-glutamyl transpeptidase in patients with malignant tumors using enzyme-linked immunosorbent assay. Cancer Res. 1985 Nov;45(11 Pt 2):5835–5839. [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wendel A. Leukotriene-mediated liver injury. Biochem Pharmacol. 1988 Jul 1;37(13):2569–2573. doi: 10.1016/0006-2952(88)90248-1. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hacker H. J., Katayama H., Masui T., Ito N., Bannasch P. Correlative histochemical studies on preneoplastic and neoplastic lesions in the kidney of rats treated with nitrosamines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(5):385–404. doi: 10.1007/BF02899047. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Moore M. A., Asamoto M., Satoh K., Tsuchida S., Sato K., Ichihara A., Ito N. Comparison of the various forms of glutathione S-transferase with glucose-6-phosphate dehydrogenase and gamma-glutamyltranspeptidase as markers of preneoplastic and neoplastic lesions in rat kidney induced by N-ethyl-N-hydroxyethylnitrosamine. Jpn J Cancer Res. 1985 Oct;76(10):919–929. [PubMed] [Google Scholar]
- Uchida T., Miyata H., Shikata T. Human hepatocellular carcinoma and putative precancerous disorders: their enzyme histochemical study. Arch Pathol Lab Med. 1981 Apr;105(4):180–186. [PubMed] [Google Scholar]
- Wapnir R. A., Mancusi V. J., Goldstein L. A. Comparative ontogenesis of gamma-glutamyl transpeptidase in rat tissues. Experientia. 1982 Jun 15;38(6):647–648. doi: 10.1007/BF01964069. [DOI] [PubMed] [Google Scholar]
- Warren B. S., Naylor M. F., Winberg L. D., Yoshimi N., Volpe J. P., Gimenez-Conti I., Slaga T. J. Induction and inhibition of tumor progression. Proc Soc Exp Biol Med. 1993 Jan;202(1):9–15. doi: 10.3181/00379727-202-43511b. [DOI] [PubMed] [Google Scholar]
- Yasutake A., Hirayama K., Inouye M. Sex difference in acute renal dysfunction induced by methylmercury in mice. Ren Fail. 1990;12(4):233–240. doi: 10.3109/08860229009060730. [DOI] [PubMed] [Google Scholar]


