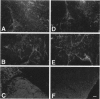
Abstract
The biological activity of transforming growth factor-beta 1 (TGF-beta) is governed by dissociation from its latent complex. Immunohistochemical discrimination of active and latent TGF-beta could provide insight into TGF-beta activation in physiological and pathological processes. However, evaluation of immunoreactivity specificity in situ has been hindered by the lack of tissue in which TGF-beta status is known. To provide in situ analysis of antibodies to differentiate between these functional forms, we used xenografts of human tumor cells modified by transfection to overexpress latent TGF-beta or constitutively active TGF-beta. This comparison revealed that, whereas most antibodies did not differentiate between TGF-beta activation status, the immunoreactivity of some antibodies was activation dependent. Two widely used peptide antibodies to the amino-terminus of TGF-beta, LC(1-30) and CC(1-30) showed marked preferential immunoreactivity with active TGF-beta versus latent TGF-beta in cryosections. However, in formalin-fixed, paraffin-embedded tissue, discrimination of active TGF-beta by CC(1-30) was lost and immunoreactivity was distinctly extracellular, as previously reported for this antibody. Similar processing-dependent extracellular localization was found with a neutralizing antibody raised to recombinant TGF-beta. Antigen retrieval recovered cell-associated immunoreactivity of both antibodies. Two antibodies to peptides 78-109 showed mild to moderate preferential immunoreactivity with active TGF-beta only in paraffin sections. LC(1-30) was the only antibody tested that discriminated active from latent TGF-beta in both frozen and paraffin-embedded tissue. Thus, in situ discrimination of active versus latent TGF-beta depends on both the antibody and tissue preparation. We propose that tissues engineered to express a specific form of a given protein provide a physiological setting in which to evaluate antibody reactivity with specific functional forms of a protein.
Full text
PDF


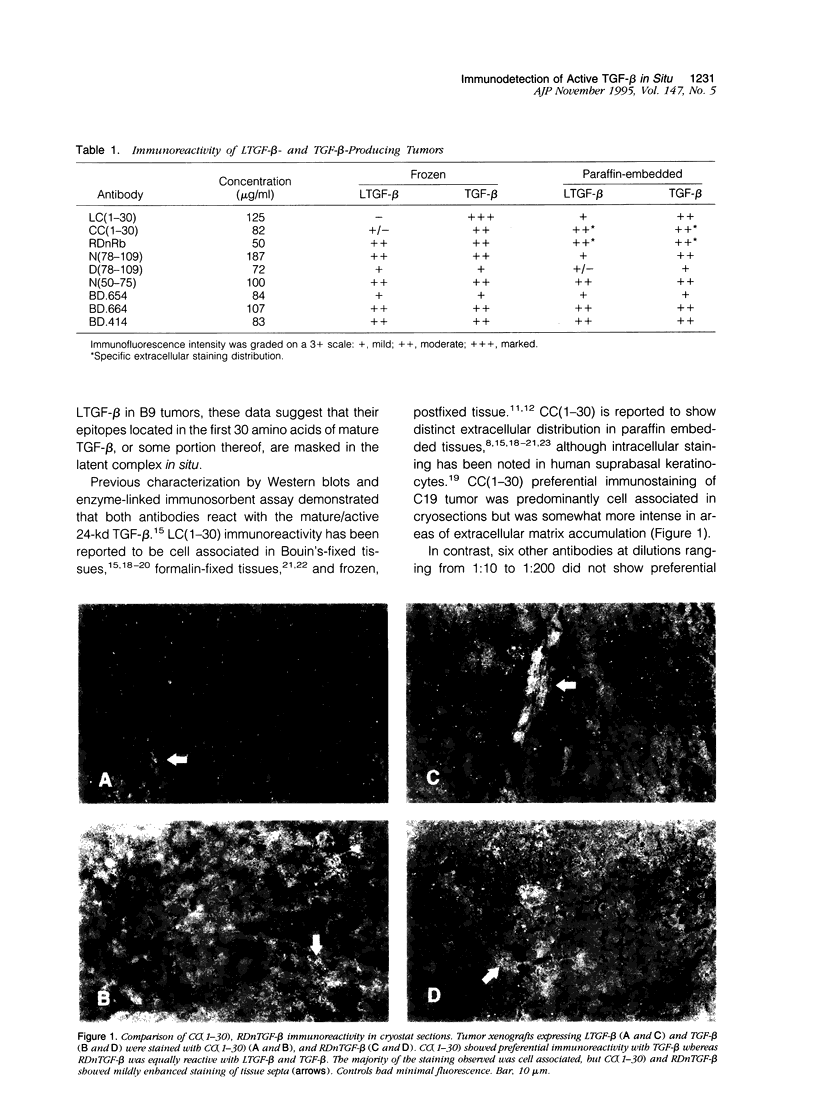
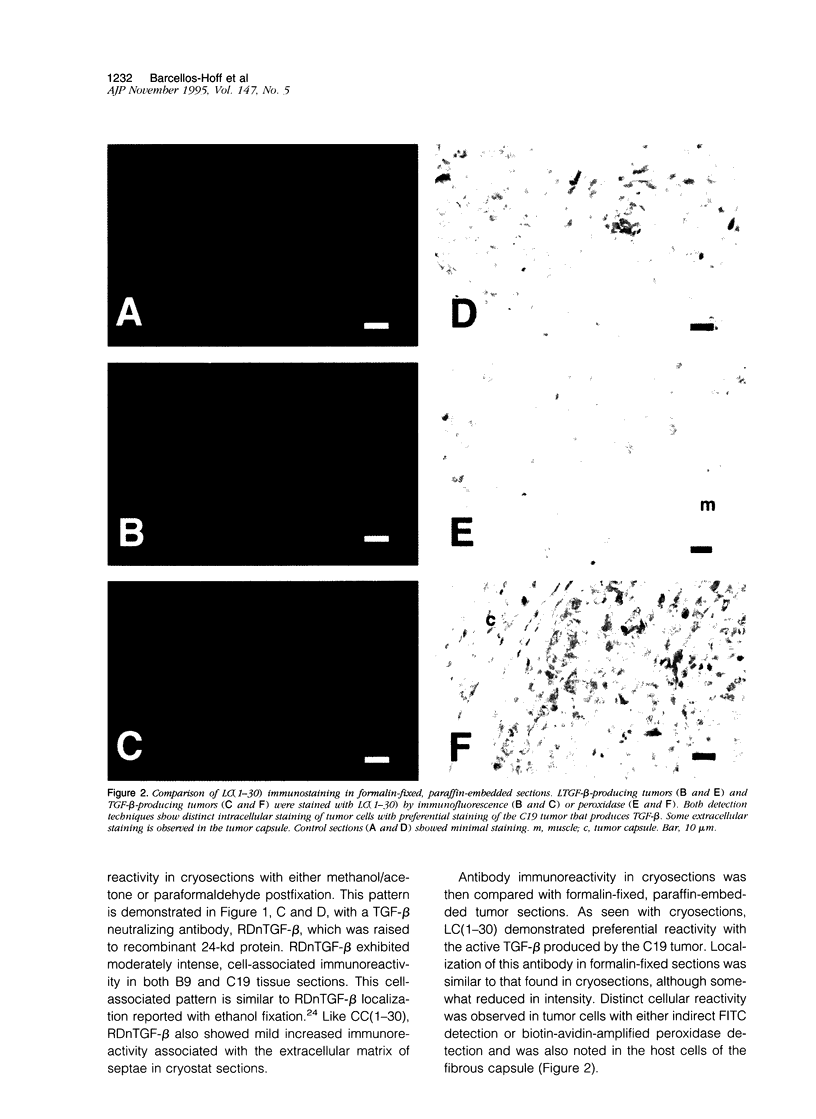
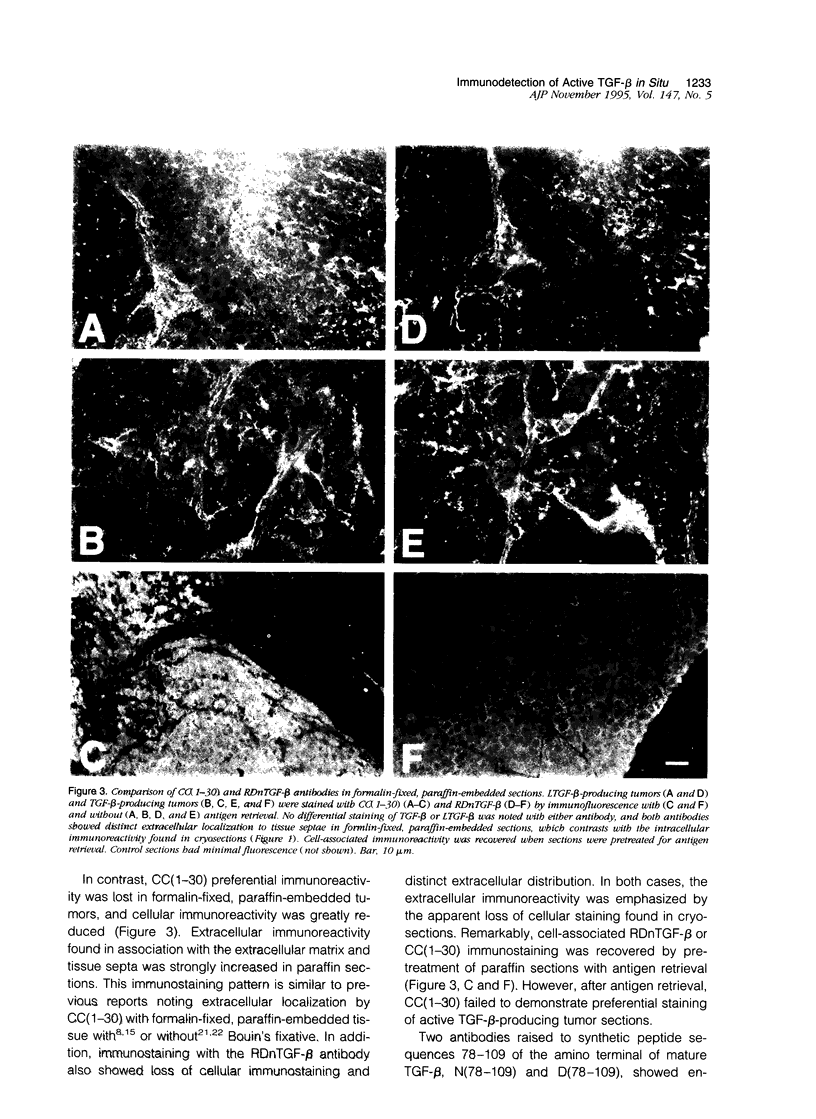



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Lopez A. R., Elfman F., Ebner R., Damsky C. H., Derynck R. Altered metabolic and adhesive properties and increased tumorigenesis associated with increased expression of transforming growth factor beta 1. J Cell Biol. 1992 Aug;118(3):715–726. doi: 10.1083/jcb.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Derynck R., Tsang M. L., Weatherbee J. A. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994 Feb;93(2):892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H. Radiation-induced transforming growth factor beta and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res. 1993 Sep 1;53(17):3880–3886. [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A. M., Marquardt H., Malacko A. R., Lioubin M. N., Purchio A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989 Aug 15;264(23):13660–13664. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Roberts A. B., Ling N., Fleurdelys B. E., Sporn M. B. Antibodies to peptide determinants in transforming growth factor beta and their applications. Biochemistry. 1988 Jan 26;27(2):739–746. doi: 10.1021/bi00402a037. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D. B. The extracellular regulation of growth factor action. Mol Biol Cell. 1992 Oct;3(10):1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlis D. J., Flanders K. C., Duffie E., Balmain A., Akhurst R. J. Discordant transforming growth factor beta 1 RNA and protein localization during chemical carcinogenesis of the skin. Cell Growth Differ. 1992 Feb;3(2):81–91. [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle R. L., Carr B. I., Scott C. D. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration. J Biol Chem. 1991 Nov 25;266(33):22444–22450. [PubMed] [Google Scholar]
- Jirtle R. L., Haag J. D., Ariazi E. A., Gould M. N. Increased mannose 6-phosphate/insulin-like growth factor II receptor and transforming growth factor beta 1 levels during monoterpene-induced regression of mammary tumors. Cancer Res. 1993 Sep 1;53(17):3849–3852. [PubMed] [Google Scholar]
- Kane C. J., Hebda P. A., Mansbridge J. N., Hanawalt P. C. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991 Jul;148(1):157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- Kane C. J., Knapp A. M., Mansbridge J. N., Hanawalt P. C. Transforming growth factor-beta 1 localization in normal and psoriatic epidermal keratinocytes in situ. J Cell Physiol. 1990 Jul;144(1):144–150. doi: 10.1002/jcp.1041440119. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher R., Jullien P., Lawrence D. A. Beta-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986 Apr 14;136(1):30–37. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- Resnick N., Zasloff M. A. Novel proteases with unusual specificities. Curr Opin Cell Biol. 1992 Dec;4(6):1032–1036. doi: 10.1016/0955-0674(92)90136-z. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Noma T., Glick A. B., Lafyatis R., Lechleider R., Jakowlew S. B., Geiser A., O'Reilly M. A., Danielpour D. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–28. doi: 10.1002/9780470514061.ch2. [DOI] [PubMed] [Google Scholar]
- Sellheyer K., Bickenbach J. R., Rothnagel J. A., Bundman D., Longley M. A., Krieg T., Roche N. S., Roberts A. B., Roop D. R. Inhibition of skin development by overexpression of transforming growth factor beta 1 in the epidermis of transgenic mice. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5237–5241. doi: 10.1073/pnas.90.11.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Williams A. O., Flanders K. C., Saffiotti U. Immunohistochemical localization of transforming growth factor-beta 1 in rats with experimental silicosis, alveolar type II hyperplasia, and lung cancer. Am J Pathol. 1993 Jun;142(6):1831–1840. [PMC free article] [PubMed] [Google Scholar]