Abstract
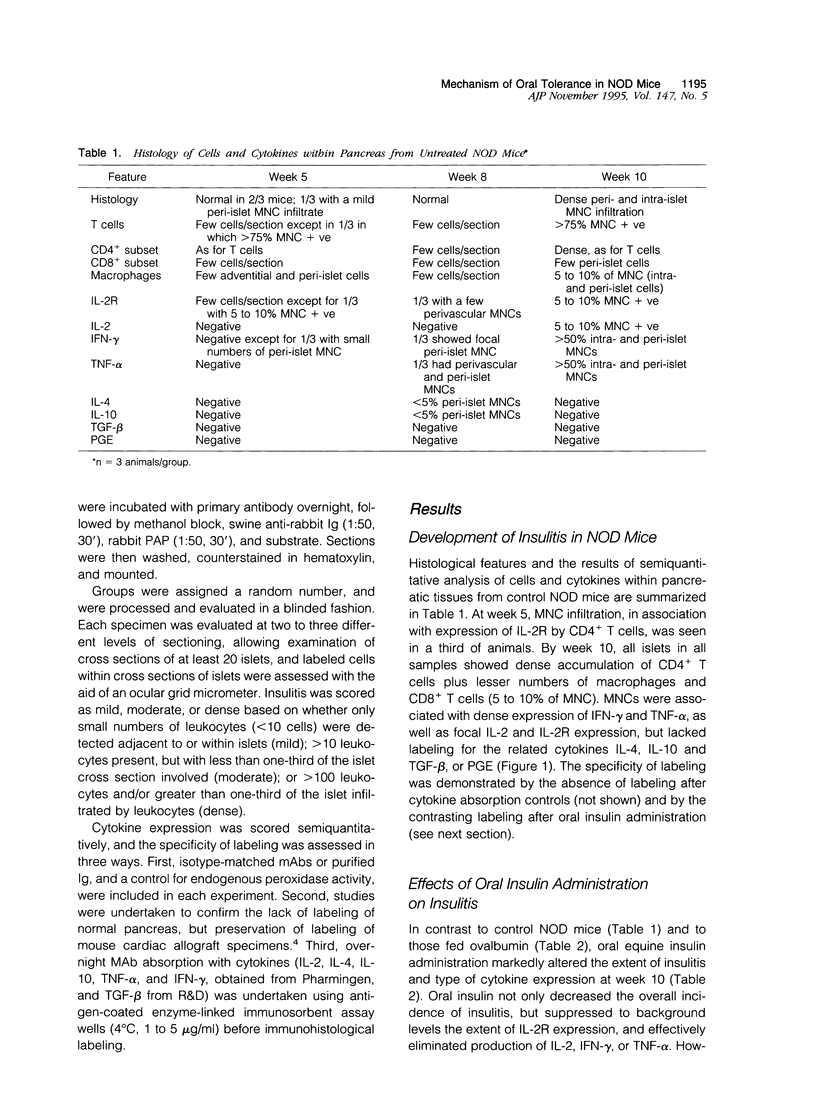
Oral administration of autoantigens suppresses development of autoimmunity in several animal models, and is being tested in clinical trials in patients with autoimmune diseases such as multiple sclerosis and rheumatoid arthritis. Non-obese diabetic (NOD) mice spontaneously develop insulin-dependent diabetes mellitus at 15 to 20 weeks of age, after mononuclear cell (MNC) infiltration of the pancreatic islets of Langerhans and destruction of insulin-producing beta cells. We have previously shown that oral administration of insulin suppresses insulitis and development of diabetes in the NOD mouse. Oral insulin has no metabolic effect on blood glucose. Oral insulin mediates its effect through a T cell-dependent mechanism as shown by adoptive transfer and T cell depletion experiments, but the mechanisms responsible have not been fully explored. We now report a serial analysis of the cells and cytokines associated with development of diabetes in NOD mice, and contrast this with the findings in animals fed equine insulin or a control protein (ovalbumin). Animals were fed 1 mg twice a week for 5 weeks, beginning at 5 weeks of age. Marked insulitis in naive or ovalbumin-fed NOD mice occurred at 10 weeks, at which time a dense peri-islet and intra-islet MNC infiltration was observed. Immunohistological studies using monoclonal antibodies showed that infiltrating MNC consisted mainly of CD4+ T cells ( > 75% of leukocytes) plus smaller numbers of macrophages and CD8+ T cells. These cells displayed evidence of immune activation with expression of receptors for interleukin-2 (IL-2R) plus Th1 cytokines; dense labeling for IFN-gamma and tumor necrosis factor-alpha, plus lesser amounts of IL-2, was observed. MNC lacked labeling for IL-4, IL-10, prostaglandin-E, or transforming growth factor-beta. By contrast, at 10 weeks, pancreatic tissues from NOD mice fed insulin showed considerably less insulitis, and the residual MNC, although still largely CD4+ T cells plus macrophages, showed dense labeling for IL-4, IL-10, prostaglandin-E, and transforming growth factor-beta and an absence of IL-2, IFN-gamma or tumor necrosis factor-alpha Taken together with our previous findings, these data indicate that oral administration of insulin affects the development of diabetes in NOD mice through the generation of cells that elaborate immunoregulatory cytokines within the target organ and shift the balance from a Th1 to a Th2 pattern of cytokine expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Maclaren N. K. Islet cell autoantigens in insulin-dependent diabetes. J Clin Invest. 1993 Oct;92(4):1608–1616. doi: 10.1172/JCI116745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerot I., Fabien N., Maguer V., Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun. 1994 Oct;7(5):655–663. doi: 10.1006/jaut.1994.1050. [DOI] [PubMed] [Google Scholar]
- Bradley B. J., Haskins K., La Rosa F. G., Lafferty K. J. CD8 T cells are not required for islet destruction induced by a CD4+ islet-specific T-cell clone. Diabetes. 1992 Dec;41(12):1603–1608. doi: 10.2337/diab.41.12.1603. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., al-Sabbagh A., Santos L. M., Das M. P., Weiner H. L. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol. 1993 Dec 15;151(12):7307–7315. [PubMed] [Google Scholar]
- Mottram P. L., Han W. R., Purcell L. J., McKenzie I. F., Hancock W. W. Increased expression of IL-4 and IL-10 and decreased expression of IL-2 and interferon-gamma in long-surviving mouse heart allografts after brief CD4-monoclonal antibody therapy. Transplantation. 1995 Feb 27;59(4):559–565. [PubMed] [Google Scholar]
- Pennline K. J., Roque-Gaffney E., Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994 May;71(2):169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation? Diabetes. 1994 May;43(5):613–621. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- Rapoport M. J., Jaramillo A., Zipris D., Lazarus A. H., Serreze D. V., Leiter E. H., Cyopick P., Danska J. S., Delovitch T. L. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993 Jul 1;178(1):87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempé P., Richard M. F., Bach J. F., Boitard C. Evidence of CD4+ regulatory T cells in the non-obese diabetic male mouse. Diabetologia. 1994 Apr;37(4):337–343. doi: 10.1007/BF00408468. [DOI] [PubMed] [Google Scholar]
- Shehadeh N. N., LaRosa F., Lafferty K. J. Altered cytokine activity in adjuvant inhibition of autoimmune diabetes. J Autoimmun. 1993 Jun;6(3):291–300. doi: 10.1006/jaut.1993.1025. [DOI] [PubMed] [Google Scholar]
- Snijdewint F. G., Kaliński P., Wierenga E. A., Bos J. D., Kapsenberg M. L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993 Jun 15;150(12):5321–5329. [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- Weiner H. L. Oral tolerance. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10762–10765. doi: 10.1073/pnas.91.23.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. D., Tisch R., Singer S. M., Cao Z. A., Liblau R. S., Schreiber R. D., McDevitt H. O. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994 Sep 1;180(3):995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. J., Davidson L., Eisenbarth G., Weiner H. L. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]



