Abstract
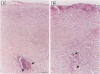
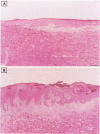
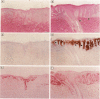
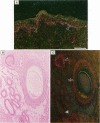
The topical application of recombinant growth factors such as epidermal growth factor, platelet-derived growth factor-BB homodimer (rPDGF-BB), keratinocyte growth factor (rKGF), and neu differentiation factor has resulted in significant acceleration of healing in several animal models of wound repair. In this study, we established highly reproducible and quantifiable full and deep partial thickness porcine burn models in which burns were escharectomized 4 or 5 days postburn and covered with an occlusive dressing to replicate the standard treatment in human burn patients. We then applied these growth factors to assess their efficacy on several parameters of wound repair: extracellular matrix and granulation tissue production, percent reepithelialization, and new epithelial area. In full thickness burns, only rPDGF-BB and the combination of rPDGF-BB and rKGF induced significant changes in burn repair. rPDGF-BB induced marked extracellular matrix and granulation tissue production (P = 0.013) such that the burn defect was filled within several days of escharectomy, but had no effect on new epithelial area or reepithelialization. The combination of rPDGF-BB and rKGF in full thickness burns resulted in a highly significant increase in extracellular matrix and granulation tissue area (P = 0.0009) and a significant increase in new epithelial area (P = 0.007), but had no effect on reepithelialization. In deep partial thickness burns, rKGF induced the most consistent changes. Daily application of rKGF induced a highly significant increase in new epithelial area (P < 0.0001) but induced only a modest increase in reepithelialization (83.7% rKGF-treated versus 70.2% control; P = 0.016) 12 days postburn. rKGF also doubled the number of fully reepithelialized burns (P = 0.02) at 13 days postburn, at least partially because of marked stimulation of both epidermal and follicular proliferation as assessed by proliferating cell nuclear antigen expression. In situ hybridization for KGFR in porcine burns revealed strong expression of KGFR on hair follicles and basal epidermis, confirming direct rKGF action on follicular as well as epidermal keratinocytes. Although the epithelial proliferation induced by rKGF resulted in marked neoepidermal psoriasiform hyperplasia with exaggerated rete ridges and neoepidermal and follicular maturation as assessed by expression of cytokeratin 10, a marker of keratinocyte terminal differentiation was not delayed and appeared to be accelerated in some rKGF-treated burns. Recombinant epidermal growth factor induced a trend toward increased new epithelial area in deep partial thickness burns, but had no effect on reepithelialization. The recombinant neu differentiation factor-alpha 2 isoform had no significant biological effects in either full or deep partial thickness burns.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carraway K. L., 3rd, Sliwkowski M. X., Akita R., Platko J. V., Guy P. M., Nuijens A., Diamonti A. J., Vandlen R. L., Cantley L. C., Cerione R. A. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994 May 13;269(19):14303–14306. [PubMed] [Google Scholar]
- Chvapil M., Speer D. P., Owen J. A., Chvapil T. A. Identification of the depth of burn injury by collagen stainability. Plast Reconstr Surg. 1984 Mar;73(3):438–441. doi: 10.1097/00006534-198403000-00018. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Danilenko D. M., Ring B. D., Lu J. Z., Tarpley J. E., Chang D., Liu N., Wen D., Pierce G. F. Neu differentiation factor upregulates epidermal migration and integrin expression in excisional wounds. J Clin Invest. 1995 Feb;95(2):842–851. doi: 10.1172/JCI117734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko D. M., Ring B. D., Yanagihara D., Benson W., Wiemann B., Starnes C. O., Pierce G. F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995 Jul;147(1):145–154. [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Deuel T. F., Kawahara R. S., Mustoe T. A., Pierce A. F. Growth factors and wound healing: platelet-derived growth factor as a model cytokine. Annu Rev Med. 1991;42:567–584. doi: 10.1146/annurev.me.42.020191.003031. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Dziewulski P. Burn wound healing: James Ellsworth Laing memorial essay for 1991. Burns. 1992 Dec;18(6):466–478. doi: 10.1016/0305-4179(92)90179-x. [DOI] [PubMed] [Google Scholar]
- Faymonville M. E., Micheels J., Bodson L., Jacquemin D., Lamy M., Adam J., Duchateau J. Biochemical investigations after burning injury: complement system, protease-antiprotease balance and acute-phase reactants. Burns Incl Therm Inj. 1987 Feb;13(1):26–33. doi: 10.1016/0305-4179(87)90252-x. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Galvin S., Loomis C., Manabe M., Dhouailly D., Sun T. T. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–300. [PubMed] [Google Scholar]
- Greenhalgh D. G., Sprugel K. H., Murray M. J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Hebda P. A., Klingbeil C. K., Abraham J. A., Fiddes J. C. Basic fibroblast growth factor stimulation of epidermal wound healing in pigs. J Invest Dermatol. 1990 Dec;95(6):626–631. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Gregory H. Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives. Mol Pharmacol. 1980 May;17(3):314–320. [PubMed] [Google Scholar]
- Housley R. M., Morris C. F., Boyle W., Ring B., Biltz R., Tarpley J. E., Aukerman S. L., Devine P. L., Whitehead R. H., Pierce G. F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994 Nov;94(5):1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Chao F. C., Stiles C. D., Antoniades H. N., Scher C. D. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979 Jun;53(6):1043–1052. [PubMed] [Google Scholar]
- Khouri R. K., Hong S. P., Deune E. G., Tarpley J. E., Song S. Z., Serdar C. M., Pierce G. F. De novo generation of permanent neovascularized soft tissue appendages by platelet-derived growth factor. J Clin Invest. 1994 Nov;94(5):1757–1763. doi: 10.1172/JCI117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara Y., Komada F., Iwakawa S., Hirai M., Fuwa T., Okumura K. Improvement in wound healing by epidermal growth factor (EGF) ointment. II. Effect of protease inhibitor, nafamostat, on stabilization and efficacy of EGF in burn. J Pharmacobiodyn. 1991 Jan;14(1):47–52. doi: 10.1248/bpb1978.14.47. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Kurihara-Bergstrom T., Woodworth M., Feisullin S., Beall P. Characterization of the Yucatan miniature pig skin and small intestine for pharmaceutical applications. Lab Anim Sci. 1986 Aug;36(4):396–399. [PubMed] [Google Scholar]
- Lavker R. M., Dong G., Zheng P. S., Murphy G. F. Hairless micropig skin. A novel model for studies of cutaneous biology. Am J Pathol. 1991 Mar;138(3):687–697. [PMC free article] [PubMed] [Google Scholar]
- Linder B. L., Chernoff A., Kaplan K. L., Goodman D. S. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4107–4111. doi: 10.1073/pnas.76.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese C., Rubin J., Ron D., Faggioni A., Torrisi M. R., Messina A., Frati L., Aaronson S. A. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: differentiation response distinguishes KGF from EGF family. J Cell Physiol. 1990 Aug;144(2):326–332. doi: 10.1002/jcp.1041440219. [DOI] [PubMed] [Google Scholar]
- Molloy R. G., O'Riordain M., Holzheimer R., Nestor M., Collins K., Mannick J. A., Rodrick M. L. Mechanism of increased tumor necrosis factor production after thermal injury. Altered sensitivity to PGE2 and immunomodulation with indomethacin. J Immunol. 1993 Aug 15;151(4):2142–2149. [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983 Jan;205(1):47–55. doi: 10.1002/ar.1092050107. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Morishima C., Deuel T. F. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991 Feb;87(2):694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B. Epidermal and dermal effects of epidermal growth factor during wound repair. J Invest Dermatol. 1990 May;94(5):624–629. doi: 10.1111/1523-1747.ep12876204. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Rubin J. S., Csaky K. G., Aaronson S. A., Mason R. J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993 Aug;92(2):969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Altrock B. W., Deuel T. F., Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991 Apr;45(4):319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A. Pharmacologic enhancement of wound healing. Annu Rev Med. 1995;46:467–481. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Yanagihara D., Mustoe T. A., Fox G. M., Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992 Jun;140(6):1375–1388. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Yanagihara D., Klopchin K., Danilenko D. M., Hsu E., Kenney W. C., Morris C. F. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994 Mar 1;179(3):831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Culouscou J. M., Whitney G. S., Green J. M., Carlton G. W., Foy L., Neubauer M. G., Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., Culouscou J. M., Carlton G. W., Rothwell V. M., Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993 Dec 2;366(6454):473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwkowski M. X., Schaefer G., Akita R. W., Lofgren J. A., Fitzpatrick V. D., Nuijens A., Fendly B. M., Cerione R. A., Vandlen R. L., Carraway K. L., 3rd Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994 May 20;269(20):14661–14665. [PubMed] [Google Scholar]
- Staiano-Coico L., Krueger J. G., Rubin J. S., D'limi S., Vallat V. P., Valentino L., Fahey T., 3rd, Hawes A., Kingston G., Madden M. R. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993 Sep 1;178(3):865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Baehner R. L. Platelet-derived growth factor promotes human peripheral monocyte activation. Blood. 1985 Jul;66(1):179–183. [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Senior R. M., Boxer L. A., Baehner R. L. Platelet-derived growth factor promotes polymorphonuclear leukocyte activation. Blood. 1984 Nov;64(5):1123–1128. [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Cardiff R., Yin S., Bikhazi N., Biltz R., Morris C. F., Pierce G. F. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994 May;144(5):862–868. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Longmuir K., Yin S., Biltz R., Morris C. F., Housley R. M., Pierce G. F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994 Mar;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Wen D., Peles E., Cupples R., Suggs S. V., Bacus S. S., Luo Y., Trail G., Hu S., Silbiger S. M., Levy R. B. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992 May 1;69(3):559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Wen D., Suggs S. V., Karunagaran D., Liu N., Cupples R. L., Luo Y., Janssen A. M., Ben-Baruch N., Trollinger D. B., Jacobsen V. L. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994 Mar;14(3):1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Elliott J. L., Matheson C., Sun J., Zhang L., Mu X., Rex K. L., Snider W. D. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993 Dec;24(12):1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- du Cros D. L. Fibroblast growth factor and epidermal growth factor in hair development. J Invest Dermatol. 1993 Jul;101(1 Suppl):106S–113S. doi: 10.1111/1523-1747.ep12363020. [DOI] [PubMed] [Google Scholar]