Abstract
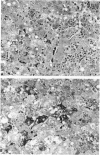
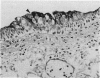
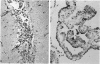
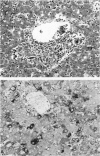
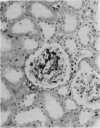
Callitrichid hepatitis is an arenavirus infection that recently emerged as a highly fatal disease of New World primates in the Callitrichidae family. As we previously reported, these primates develop hepatitis after contact with mice that are infected with variants of LCMV (LVMCCH), recently determined to have 86% identity with GC-P gene of the Armstrong and Western strains of LCMV. Here, we describe the histopathological lesions and tissue localization of viral antigens in confirmed cases of callitrichid hepatitis from recent outbreaks in two U.S. zoos. The liver in marmosets and tamarins with fatal infections consistently showed degeneration, necrosis, and inflammation, with variable involvement of the spleen, lymph nodes, adrenal glands, intestine, pancreas, and central nervous system. Lymphocytic choriomeningitis virus antigens were identified immunohistochemically in necrotic foci in these organs as well as in nondegenerating areas in lungs, kidney, urinary bladder, brain, and testes. The multi-organ tropism and histological pattern of LCMV infection in marmosets and tamarins are similar to those reported for the highly virulent arenavirus that causes Lassa fever in humans. Comparative studies of callitrichid hepatitis and Lassa fever would therefore be mutually beneficial for human and nonhuman primate medicine.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Hahn C. S., Somasundaram T., Villarete L., Matloubian M., Strauss J. H. Molecular basis of organ-specific selection of viral variants during chronic infection. J Virol. 1991 Aug;65(8):4242–4247. doi: 10.1128/jvi.65.8.4242-4247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis R. T., Jahrling P. B., DePaoli A. Pathology of Lassa virus infection in the rhesus monkey. Am J Trop Med Hyg. 1982 Sep;31(5):1038–1045. doi: 10.4269/ajtmh.1982.31.1038. [DOI] [PubMed] [Google Scholar]
- Childs J. E., Glass G. E., Korch G. W., Ksiazek T. G., Leduc J. W. Lymphocytic choriomeningitis virus infection and house mouse (Mus musculus) distribution in urban Baltimore. Am J Trop Med Hyg. 1992 Jul;47(1):27–34. doi: 10.4269/ajtmh.1992.47.27. [DOI] [PubMed] [Google Scholar]
- Connolly B. M., Jenson A. B., Peters C. J., Geyer S. J., Barth J. F., McPherson R. A. Pathogenesis of Pichinde virus infection in strain 13 guinea pigs: an immunocytochemical, virologic, and clinical chemistry study. Am J Trop Med Hyg. 1993 Jul;49(1):10–24. doi: 10.4269/ajtmh.1993.49.10. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Gregg M. B. Recent outbreaks of lymphocytic choriomeningitis in the United States of America. Bull World Health Organ. 1975;52(4-6):549–553. [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Hesse R. A., Rhoderick J. B., Elwell M. A., Moe J. B. Pathogenesis of a pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect Immun. 1981 May;32(2):872–880. doi: 10.1128/iai.32.2.872-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Peters C. J. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch Pathol Lab Med. 1992 May;116(5):486–488. [PubMed] [Google Scholar]
- Jahrling P. B., Smith S., Hesse R. A., Rhoderick J. B. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun. 1982 Aug;37(2):771–778. doi: 10.1128/iai.37.2.771-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguens R. P., Gonzalez P. H., Ponzinibbio C., Chambo J. Damage of human polymorphonuclear leukocytes by Junin virus. Med Microbiol Immunol. 1986;175(2-3):177–180. doi: 10.1007/BF02122444. [DOI] [PubMed] [Google Scholar]
- Lucia H. L., Coppenhaver D. H., Harrison R. L., Baron S. The effect of an arenavirus infection on liver morphology and function. Am J Trop Med Hyg. 1990 Jul;43(1):93–98. doi: 10.4269/ajtmh.1990.43.93. [DOI] [PubMed] [Google Scholar]
- Montali R. J., Ramsay E. C., Stephensen C. B., Worley M., Davis J. A., Holmes K. V. A new transmissible viral hepatitis of marmosets and tamarins. J Infect Dis. 1989 Nov;160(5):759–765. doi: 10.1093/infdis/160.5.759. [DOI] [PubMed] [Google Scholar]
- Montali R. J., Scanga C. A., Pernikoff D., Wessner D. R., Ward R., Holmes K. V. A common-source outbreak of callitrichid hepatitis in captive tamarins and marmosets. J Infect Dis. 1993 Apr;167(4):946–950. doi: 10.1093/infdis/167.4.946. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. The arenaviruses--an introduction. Curr Top Microbiol Immunol. 1987;133:1–4. [PubMed] [Google Scholar]
- Peters C. J., Jahrling P. B., Liu C. T., Kenyon R. H., McKee K. T., Jr, Barrera Oro J. G. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- Pulkkinen A. J., Pfau C. J. Plaque size heterogeneity: a genetic trait of lymphocytic choriomeningitis virus. Appl Microbiol. 1970 Jul;20(1):123–128. doi: 10.1128/am.20.1.123-128.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R., de Manzione N., Tesh R. B., Rico-Hesse R., Shope R. E., Betancourt A., Godoy O., Bruzual R., Pacheco M. E., Ramos B. Venezuelan haemorrhagic fever. Lancet. 1991 Oct 26;338(8774):1033–1036. doi: 10.1016/0140-6736(91)91899-6. [DOI] [PubMed] [Google Scholar]
- Stephensen C. B., Jacob J. R., Montali R. J., Holmes K. V., Muchmore E., Compans R. W., Arms E. D., Buchmeier M. J., Lanford R. E. Isolation of an arenavirus from a marmoset with callitrichid hepatitis and its serologic association with disease. J Virol. 1991 Aug;65(8):3995–4000. doi: 10.1128/jvi.65.8.3995-4000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephensen C. B., Park J. Y., Blount S. R. cDNA sequence analysis confirms that the etiologic agent of callitrichid hepatitis is lymphocytic choriomeningitis virus. J Virol. 1995 Feb;69(2):1349–1352. doi: 10.1128/jvi.69.2.1349-1352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R. B., Jahrling P. B., Salas R., Shope R. E. Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg. 1994 Apr;50(4):452–459. doi: 10.4269/ajtmh.1994.50.452. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Johnson K. M., Lange J. V., Gardner J. J., Kiley M. P., McCormick J. B. Experimental infection of rhesus monkeys with Lassa virus and a closely related arenavirus, Mozambique virus. J Infect Dis. 1982 Sep;146(3):360–368. doi: 10.1093/infdis/146.3.360. [DOI] [PubMed] [Google Scholar]
- Walker D. H., McCormick J. B., Johnson K. M., Webb P. A., Komba-Kono G., Elliott L. H., Gardner J. J. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982 Jun;107(3):349–356. [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Murphy F. A. Pathology and pathogenesis of arenavirus infections. Curr Top Microbiol Immunol. 1987;133:89–113. doi: 10.1007/978-3-642-71683-6_7. [DOI] [PubMed] [Google Scholar]
- Weissenbacher M. C., Coto C. E., Calello M. A., Rondinone S. N., Damonte E. B., Frigerio M. J. Cross-protection in nonhuman primates against Argentine hemorrhagic fever. Infect Immun. 1982 Feb;35(2):425–430. doi: 10.1128/iai.35.2.425-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki S. R., Greer P. W., Coffield L. M., Goldsmith C. S., Nolte K. B., Foucar K., Feddersen R. M., Zumwalt R. E., Miller G. L., Khan A. S. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995 Mar;146(3):552–579. [PMC free article] [PubMed] [Google Scholar]