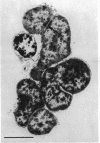
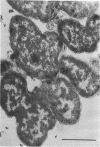
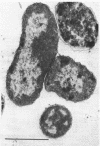
Abstract
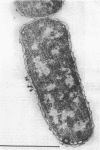
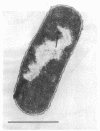
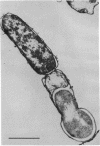
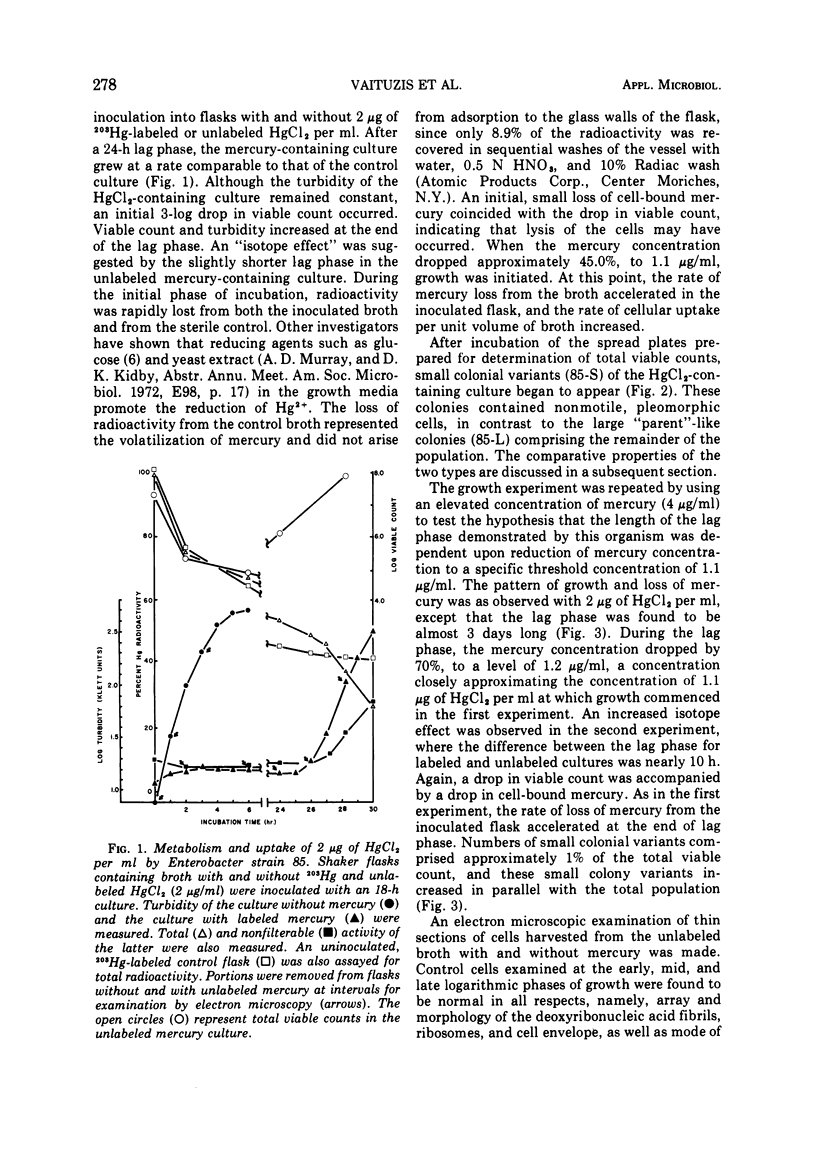
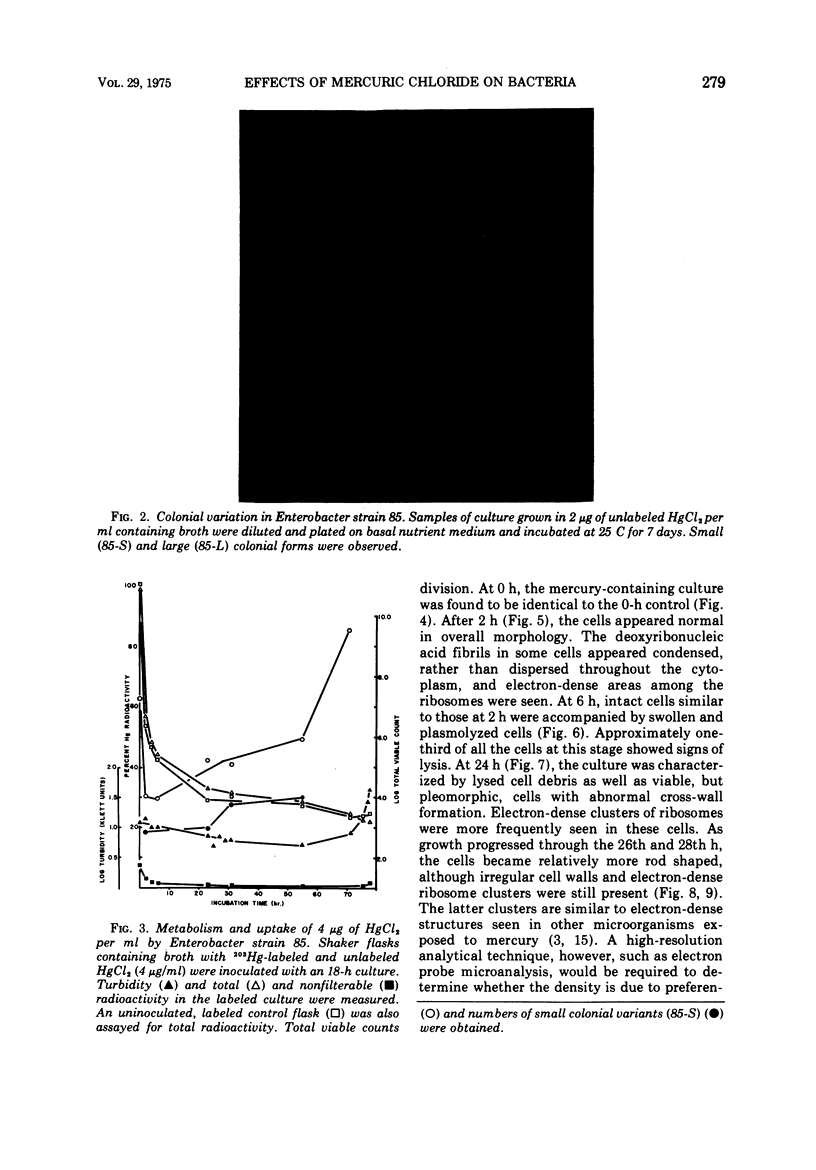
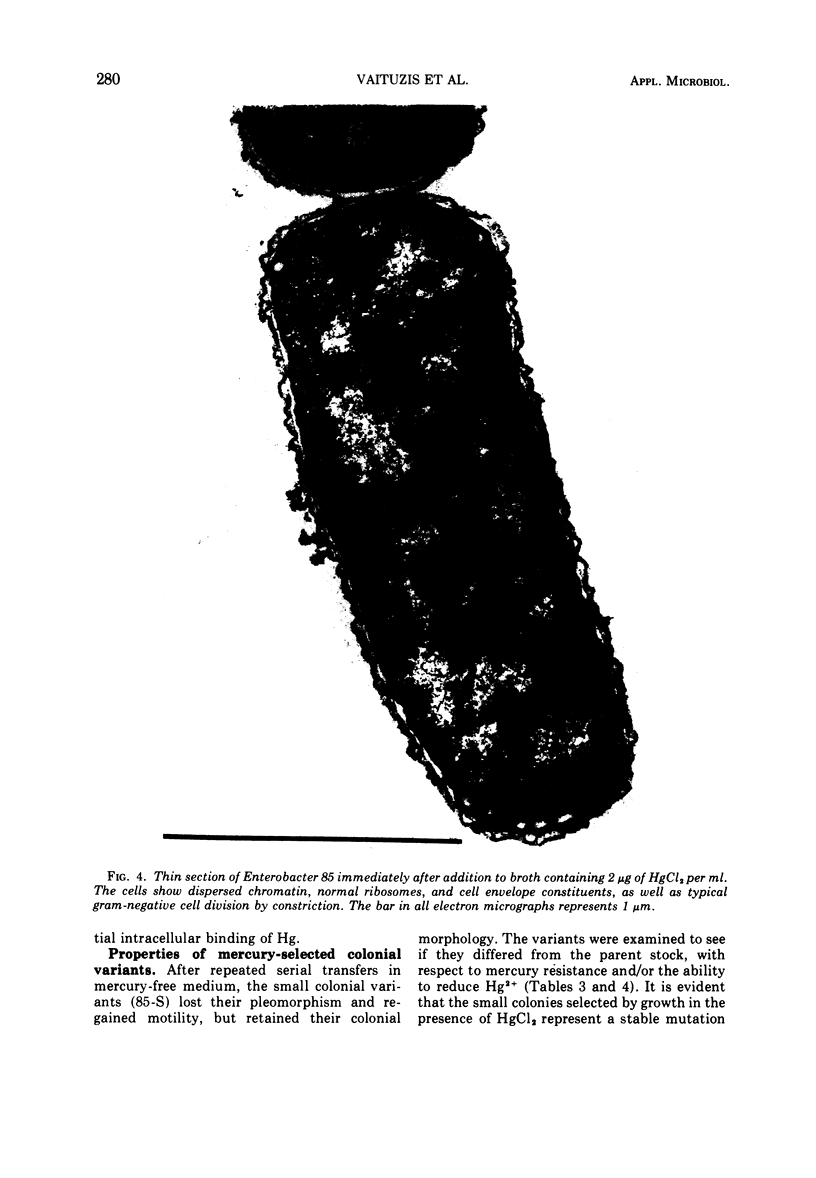
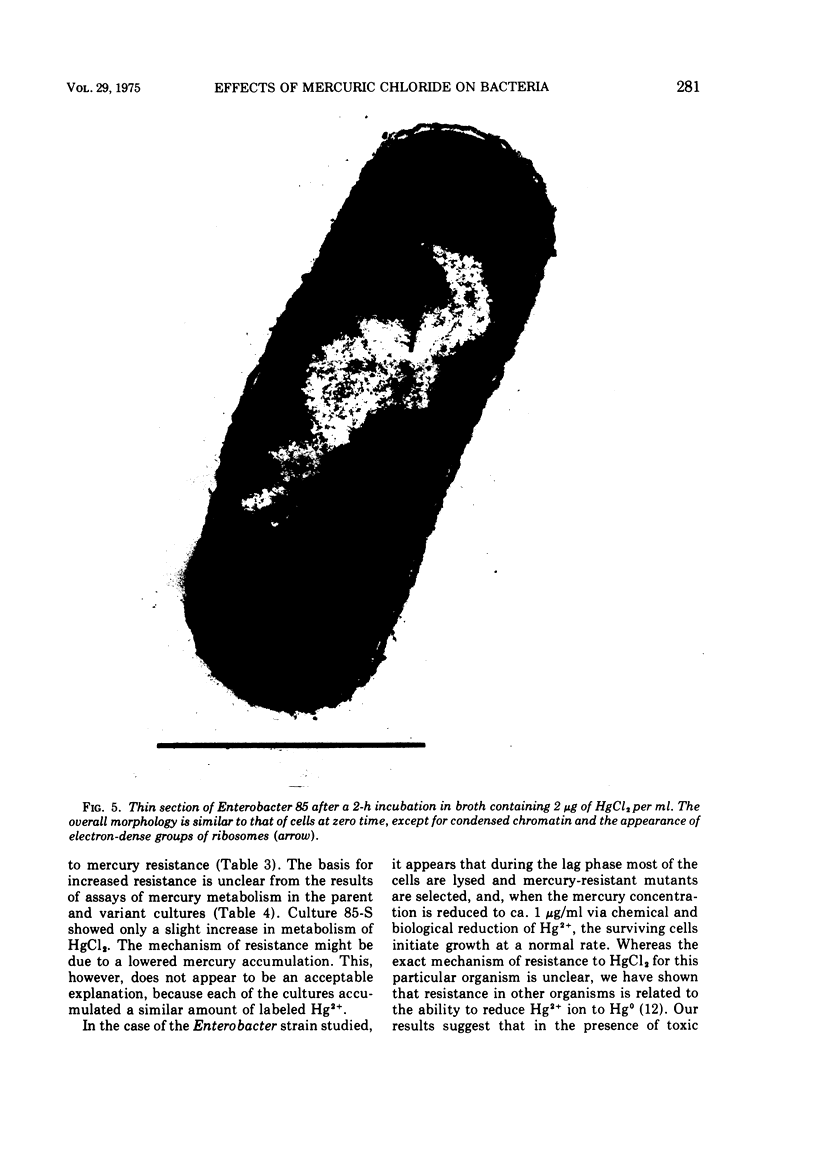
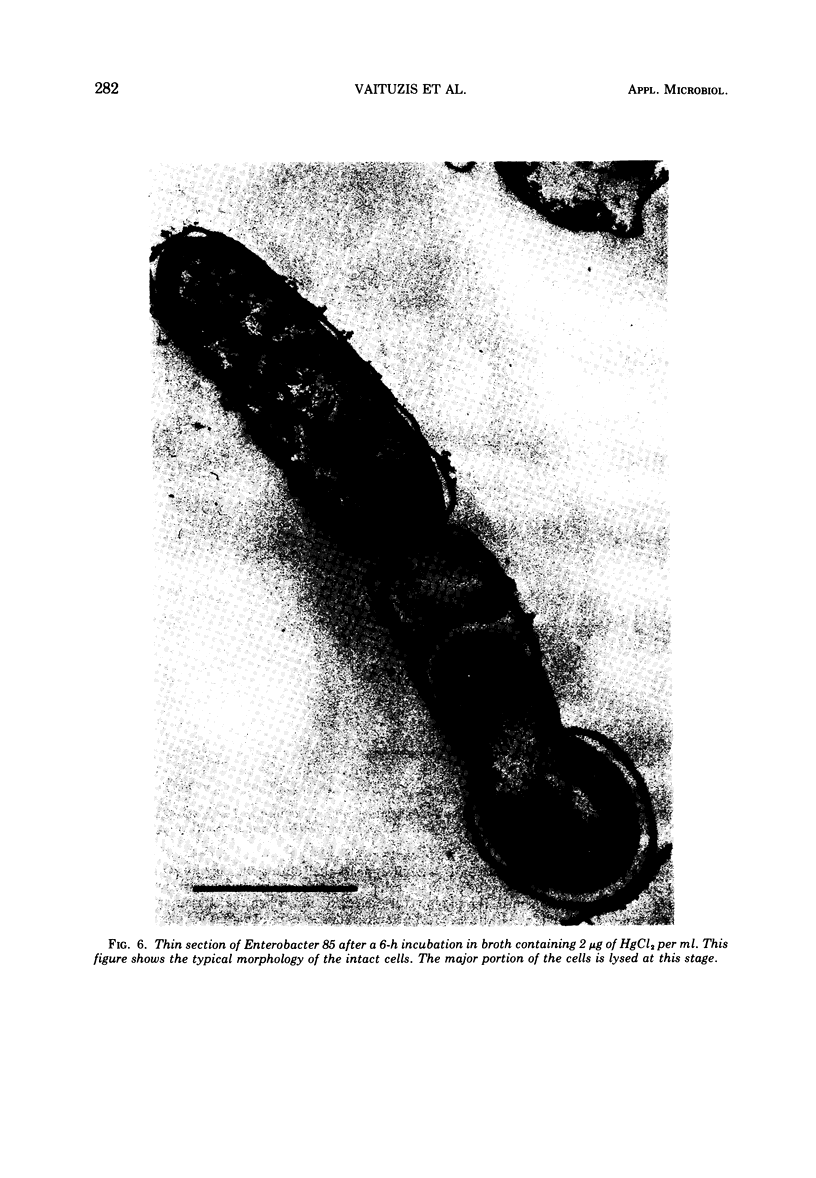
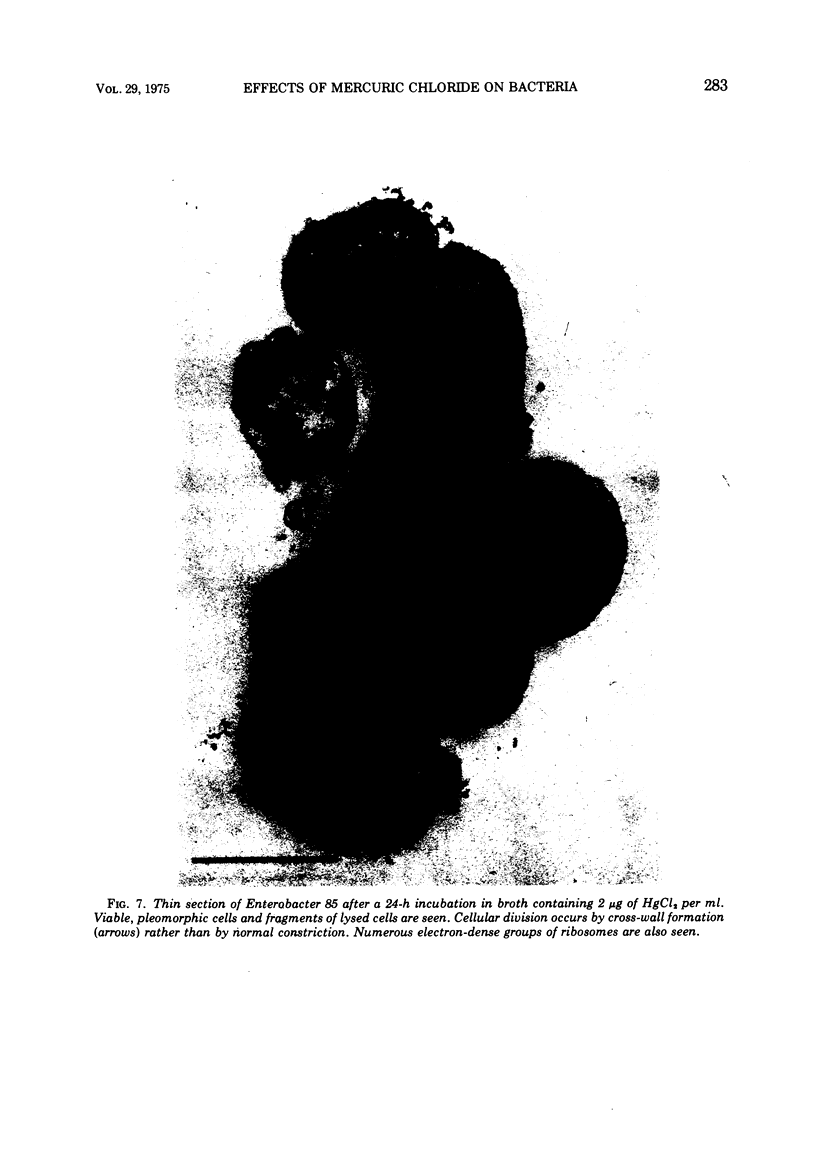
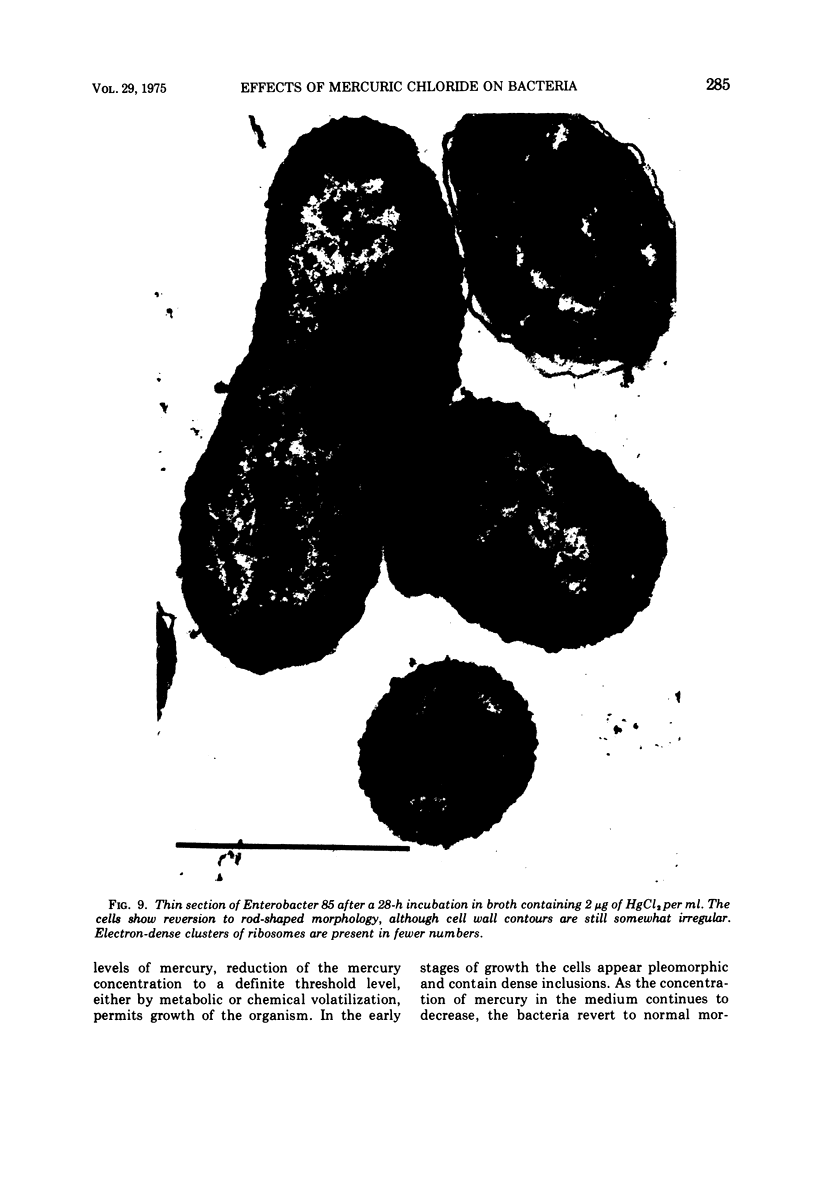
A survey of the comparative cytological effects of growth in the presence of mercury by a group of mercury-resistant bacterial cultures and a characterization of the process of bacterial adaptation to Hg2+ ion was accomplished. Mercury resistance was found to be dependent upon the ability to volatilize mercury from the medium and upon the amount of mercury accumulated by the cells. The results indicate that most cultures which adapt to growth in the presence of HgCl2 exhibit extensive morphological abnormalities. Significant effects are delay in the onset of growth and cell division and numerous structural irregularities associated with cell wall and cytoplasmic membrane synthesis and function. A detailed analysis of the adaptation process and the resulting effects on morphology was performed on an Enterobacter sp. During the period preceding active multiplication, a selection for mercury-resistant mutants occurred. It was also demonstrated that growth commenced only at a specific threshold concentration of Hg2+.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat D., Shelef G., Gruner N., Shuval H. I. Growth of Chlamydomonas in a medium containing mercury. Nature. 1972 Nov 3;240(5375):43–44. doi: 10.1038/240043a0. [DOI] [PubMed] [Google Scholar]
- Bernheim F. The effect of cyanogen iodide and mercuric chloride on the permeability of cells of Pseudomonas aeruginosa and the antagonistic action of sulfhydryl compounds. Proc Soc Exp Biol Med. 1971 Nov;138(2):444–447. doi: 10.3181/00379727-138-35916. [DOI] [PubMed] [Google Scholar]
- Brunker R. L., Bott T. L. Reduction of mercury to the elemental state by a yeast. Appl Microbiol. 1974 May;27(5):870–873. doi: 10.1128/am.27.5.870-873.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura I., Funaba T., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. II. NADPH-dependent reduction of mercuric chloride and vaporization of mercury from mercuric chloride by a multiple drug resistant strain of Escherichia coli. J Biochem. 1971 Dec;70(6):895–901. doi: 10.1093/oxfordjournals.jbchem.a129719. [DOI] [PubMed] [Google Scholar]
- Komura I., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. I. Vaporization of a mercury compound from mercuric chloride by multiple drug resistant strains of Escherichia coli. J Biochem. 1971 Dec;70(6):885–893. doi: 10.1093/oxfordjournals.jbchem.a129718. [DOI] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzi R. Toxicity of mercury to phytoplankton. Nature. 1972 May 5;237(5349):38–40. doi: 10.1038/237038a0. [DOI] [PubMed] [Google Scholar]
- STUTZENBERGER F. J., BENNETT E. O. SENSITIVITY OF MIXED POPULATIONS OF STAPHYLOCOCCUS AUREUS AND ESCHERICHIA COLI TO MERCURIALS. Appl Microbiol. 1965 Jul;13:570–574. doi: 10.1128/am.13.4.570-574.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J Bacteriol. 1973 Feb;113(2):1070–1072. doi: 10.1128/jb.113.2.1070-1072.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle L. E., Pavlat W. A., Cameron I. L. Sublethal cytotoxic effects of mercuric chloride on the ciliate Tetrahymena pyriformis. J Protozool. 1973 May;20(2):301–304. doi: 10.1111/j.1550-7408.1973.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Tonomura K., Maeda K., Futai F., Nakagami T., Yamada M. Stimulative vaporization of phenylmercuric acetate by mercury-resistant bacteria. Nature. 1968 Feb 17;217(5129):644–646. doi: 10.1038/217644b0. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]