Abstract
It has been demonstrated recently that coagulation factor XIII (FXIII) plays an extraordinary role in myocardial healing after infarction, improving survival in a mouse model. Common FXIII gene variants (i.e. FXIIIA-V34L and FXIIIB-H95R) significantly influence the molecular activity. To evaluate whether there is a relationship between the two FXIII gene variants and survival in patients after myocardial infarction (MI), V34L and H95R were PCR-genotyped in a cohort of 560 MI cases and follow-up was monitored. Cases with ST-segment elevation MI (STEMI) were 416 (74.3%) and 374 of these were treated with primary percutaneous coronary intervention (PCI) (89.9%). The remaining 144 patients showed non-ST-segment elevation MI (NSTEMI) at enrollment. The combined endpoint was the occurrence of death, re-infarction, and heart failure. Kaplan-Meier analysis at one year yielded an overall rate for adverse events of 24.5% with a lower incidence in the L34-carriers (28.8% vs 17.1%; log-rank, P = 0.00025), similar to that of the 416 STEMI (23.8%) being (28.0% and 16.9%; VV34- and L34-carriers respectively; log-rank, P = 0.001). Primary PCI-group had a slight lower incidence (22.9%) of adverse events (26.8% and 17.1%; VV34- and L34-carriers respectively; log-rank, P = 0.009). During hospitalization, 506 patients received PCI (374 primary PCI and 132 elective PCI). Significance was conserved also in the overall PCI-group (28.6% and 17.8%; VV34- and L34-carriers respectively; log-rank, P = 0.001). Similar findings were observed at 30 days follow-up. Cases carrying both FXIII variants had improved survival rate (log-rank, P = 0.019). On the other hand, minor bleeding complications were found increased in L34-carriers (P = 0.0001) whereas major bleeding complications were not. Finally, more direct evidence on the role of FXIII molecule on survival might come from the fact that despite significant FXIII antigen reductions observed in cases after MI, regardless the FXIII genotype considered, L34-carriers kept almost normal FXIII activity (VV34- vs L34-carriers; P < 0.001). We conclude that FXIII L34-allele improves survival after MI in all the groups analyzed, possibly through its higher activity associated with assumable positive effects on myocardial healing and recovered functions. Genetically determined higher FXIII activity might influence post-MI outcome. This paves the way for using FXIII molecules to improve myocardial healing, recovery of functions, and survival after infarction.
INTRODUCTION
Recently, low circulating levels of coagulation factor XIII (FXIII) were associated with the worst clinical outcome after myocardial infarction (MI) injury in a mouse model by impaired myocardial healing (1). In neonatal cardiac allograft model, graft viability and contractile performance were higher in FXIII-injected animals (2). Previous reports ascribed novel pro-angiogenic effects to FXIII molecule in vitro and in vivo (3,4). Key roles of FXIII in wound healing and tissue repair are strongly suggested by numerous observations, starting from its role in improving healing of cutaneous lesions to the beneficial effects on cell migration into the wound (5,6). We recently reported cases with chronic skin lesions had inverse association of the wound area with FXIII activity and that the lesion extension and progression increased as the number of a FXIII polymorphic allele decreased in the genotype of patients (7). In addition, carrier patients with chronic vascular insufficiency and venous leg ulcer had a significantly shorter mean healing time of the skin lesion after venous reflux correction. This happened despite the fact that the elective vascular surgery intervention was completely successful in all treated patients (8). Reperfusion treatment by percutaneous coronary intervention (PCI) is the most efficacious and utilized medical strategy in acute MI (9,10). Despite this and recent developments, the frequency of adverse cardiac events remains a significant clinical problem, limiting the overall long-term survival (11,12). Conventional acquired and circumstantial situations affecting survival after successful PCI have been thoroughly investigated (11–13), but the role of genetic markers is still poorly defined. A recent study failed in associating prediction for adverse cardiac events after successful mechanical reperfusion to classical genetic thrombophilic markers (14), while two studies found FXIIIA L34-allele related to a reduced reperfusion efficacy after thrombolysis (15,16). FXIII plays a pivotal role in the thrombus formation/organization, circulates in plasma as an inactive hetero-tetramer of two catalytic A-subunits and two accessory B-subunits (A2B2) and is encoded by different genes (17,18). FXIII is activated (FXIIIa) by thrombin, which cleaves the A-subunit at R37-G38 releasing the activation peptide, favoring in turn the B-subunit dissociation, an essential step to express a complete enzyme activity. FXIIIa catalyzes the cross-linking between fibrin molecules, rendering a thrombus more stable and resistant, suggesting a role in the pathogenesis of atherothrombotic diseases (17–19). In addition, several extracellular matrix (ECM) components of diverse tissues are specific substrates for FXIII (17). A G > T transversion in codon 34 of FXI-IIA-subunit (FXIIIA-V34L), three amino acids from the thrombin activation site, influences the activation rate of the molecule (20), the fibrin clot structure (21), and confers protection against MI (22–24), although not all studies reported such a protection (25–27). An A > G transition in codon 95 of FXIIIB-subunit (FXI-IIB-H95R), in the second sushi domain of the molecule, was associated with increased dissociation rate of the A2B2 tetramer (28) and with a weak MI risk reduction (29,30). In addition, it was found a synergistic risk reduction when R95 was in combination with the L34-allele (29). FXIII molecule activity strongly depends from intragenic polymorphisms (31). The V34L is considered as the main functional locus (32) with stronger L34-associated increased activity being in L34-homozyogotes basically twice as high as VV34-wild-types (7,33). The prevalence of these polymorphisms is high in Caucasians (20–30), and no clear explanations exist for why variants at increased activity should be protective against atherothrombosis. Thus, in a cohort of 560 cases with acute MI, we tested the hypotheses that two functional FXIII gene variants (i.e. V34L and H95R) may have prognostic value after MI, speculating that higher FXIII activity may have positive effects on survival by improved myocardial healing.
METHODS AND SUBJECTS
Definition of Patients
From March 2002 to December 2005, we recruited 560 acute MI patients (whole group; mean age 66.5 ± 12.1 years; 73.6% men) admitted to the Unit Coronary Care (UCC) of the University-Hospital of Ferrara. Inclusion criteria were: prolonged chest pain occurring at rest accompanied by electrocardiography (ECG) ischemic changes (ST-segment elevation or depression ≥ 1 mm, T-waves inversion, flat T-waves, pseudo-normalization of previously negative T-waves). Myocardial necrosis was defined when the serum CK-MB and/or Troponin-I values were greater than the upper reference limit (respectively, 5.0 ng/mL and 0.1 ng/mL) in two separate blood samples.
The number of patients showing ST-segment elevation myocardial infarction at enrollment (STEMI group) was 416 (74.3%). The remaining 144 patients (25.7%) did not show ST-segment elevation myocardial infarction at enrollment (NSTEMI group). A total of 521 patients (93%) underwent coronary angiography during the hospitalization. Coronary artery disease was defined on the basis of angiographic criteria as stenosis > 50% in a major coronary artery or major branch, with classification according to the number of affected arteries. Of note, 374 patients (89.9%) of STEMI group were treated at entry with primary percutaneous coronary intervention (primary-PCI group). Of 42 STEMI patients not submitted to primary PCI, 26 (62.0%) underwent coronary angiography and 19 (45.2%) received PCI. In the NSTEMI group, coronary angiography was performed in 121 (84.0 %) patients, and 113 (78.5%) were treated with PCI. Overall, 506 (90.3%) patients were treated during the hospitalization with PCI (overall-PCI group).
Patients received standard routine medical therapy that included aspirin, clopidogrel, glycoprotein IIb/IIIa inhibitors, unfractioned or low molecular weight heparin, β-blockers, ACE-inhibitors, statins, and nitrates, with dosages and timing of administration according to current American and European guidelines (34). Pharmacological therapies at discharge consisted of aspirin (91%), thienopyridines (89%), ACE-inhibitors (92%), β-blockers (80%), statins (97%), nitrates (17%), and diuretics (30%). Clinical features of patients are indicated in Table 1. All cases come from northern Italy and have a similar ethnic background. All recruited patients gave written informed consent to enter the study, which was approved by the local Ethics Committee.
Table 1.
Baseline Characteristics of MI cases
| Characteristics | All cases (n = 560) | STEMI (n = 416) | NSTEMI (n = 144) | P |
|---|---|---|---|---|
| Age (y ± SD) | 66.5 ± 12.1 | 66.2 ± 12.3 | 67.5 ± 11.4 | NS |
| Males (n, %) | 412 (73.6) | 305 (73.3) | 107 (74.3) | NS |
| Hypertension (n, %) | 321 (57.3) | 226 (54.3) | 95 (66.0) | 0.003 |
| Dyslipidemia (n, %) | 336 (60.0) | 241 (60.0) | 95 (65.9) | NS |
| Diabetes (n, %) | 120 (21.4) | 78 (18.7) | 42 (29.0) | 0.03 |
| Smokers (n, %) | 253 (45.2) | 198 (47.6) | 55 (38.1) | 0.002 |
| Cardiovascular history | ||||
| aCABG (n, %) | 34 (6.1) | 22 (5.3) | 12 (8.3) | NS |
| bPCI (n, %) | 88 (15.7) | 55 (13.2) | 33 (22.9) | 0.03 |
| cMI (n, %) | 104 (18.6) | 61 (14.7) | 43 (29.9) | 0.001 |
| Presentation profile | ||||
| Systolic pressure, mmHg | 125.4 ± 20 | 126.0 ± 23 | 124.1 ± 17 | NS |
| Heart rate, bpm | 83 ± 20 | 84 ± 23 | 80 ± 13 | NS |
| Killip class > 1 (n, %) | 70 (12.5) | 60 (14.4) | 10 (6.9) | 0.04 |
| dVessel disease | ||||
| One (n, %) | 246 (47.2) | 192 (48.0) | 54 (44.6) | NS |
| Two (n, %) | 165 (31.7) | 124 (31.0) | 41 (33.9) | NS |
| Three (n, %) | 110 (21.1) | 84 (21.0) | 26 (21.5) | NS |
CABG = coronary artery bypass graft.
PCI = percutaneous coronary intervention.
MI = previous myocardial infarction.
coronarography was performed in 521 patients.
FXIII Level Measurement
FXIII activity was measured by a commercial method (Berichrom Factor XIII, Dade Behring, Marburg, Germany) as previously reported (35). Briefly, the main reagents and assay conditions were: 40 μL of undiluted plasma sample; 400 μL of reagent mixture (bovine thrombin 5.0 U/mL, CaCl2 0.6 g/L; NADH 0.25 g/L, synthetic peptide as FXIII substrate 1.2 g/L); 180 s of activation time at 37°C. FXIII activity was determined after 300 s by reading at 340 nm by an automated coagulation analyzer (BCT, Dade Behring Coagulation Timer; Dade Behring, Marburg, Germany). (For detailed kit components see also code OWSU G11 C0542 at www.dadebehring.com.) Values of FXIII activity ranging from 65 to 120% were considered normal.
FXIIIA subunit antigen levels were measured by Laurell immuno-electrophoresis as previously described (35) with the exception that the specific FXI-IIA antiserum utilized (1.2% in 1% standard agarose gel in tristricine-EDTA buffer, pH = 8.5) was from Diagnostica Stago (Assera XIII-A, Stago, Asnières, France).
Genotype Analysis
Blood was collected at admission to avoid loss of cases. Genomic DNA was obtained from peripheral blood, and genotyping for the FXIIIA-V34L and FXI-IIB-H95R polymorphisms was performed by PCR-amplification followed by specific restriction enzyme digestion (7,30). The forward and reverse amplification primers for FXIIIA were respectively, 5′-CATGCCTTTTCTGTTGTCTTC and 5′-TACCTTGCAGGTTGACGCCCCGGGG CACTA. The underlined base was changed from the native sequence to introduce a restriction DdeI site and the L34-allele results in digestion of the native PCR product (192 bp). The PCR conditions for FXIIIA were as follows: 5 min initial at 94°C, followed by 30 cycles of 94°C for 30 s, 50°C for 25 s, and 72°C for 60 s. The forward and reverse amplification primers for FXIIIB were respectively, 5′-AAAGACAAGCTTAGTTTCATCATT and 5′-TCTTCAGTTTAGGAAATGAT TCTTAT and the R95-allele alters a restriction NsiI site in the native PCR product (264 bp). The PCR conditions for FXI-IIB were as follows: 5 min initial at 94°C, followed by 30 cycles of 94°C for 60 s, 57°C for 60 s, and 72°C for 90 s. All PCR cycles were performed in a Peltier Thermal Cycler apparatus (PTC-200; M. J. Research, Inc., Watertown, MA) and were completed with a 5 min final extension step at 72°C. DNA digestions were performed according to suppliers’ instructions and the digested products were analyzed on 8.5% PAGE stained by ethidium bromide. Confirmation of genotypes was carried out by regenotyping a random selection of samples for each polymorphism investigated. There were no discrepancies between genotypes determined in duplicate.
Follow-up and Description of Endpoint
The primary endpoint was a composite of cardiovascular death, re-infarction and heart failure at 30-days and one-year (mean follow-up 311 ± 81 days; range 240–415). No patients were lost to follow-up. Cardiovascular origin of death was established clinically or at autopsy. Reinfarction was diagnosed in the presence of new ischemic symptoms and recurrent elevation of biochemical myocardial necrosis markers (CK-MB and/or Troponin-I that were in the upper part of normal range in two separate blood samples) with or without concurrent ECG changes. Occurrence of heart failure required the presence of rest or effort dyspnea and ≥ 1 of the following: pulmonary rales at lung auscultation, S3 tone, evidence of pulmonary congestion at chest x-ray, new appearance of peripheral edema, or use of diuretics. Bleeding complications were defined according to the criteria of TIMI trials (36). Bleeding was defined as major when it was intracranial, retroperitoneal, intraocular, with any hemoglobin loss ≥ 5 g/dL (or hematocrit reduction ≥ 15%), or when it required blood transfusion. Any other bleeding was defined as minor.
Statistical Analysis
Continuous data were presented as means ± SD, with the significance of differences judged by t-test. Categorical variables were summarized in terms of number and percentages. Fisher’s exact test (two-tailed) was used as appropriate. Survival curves were constructed by the Kaplan-Meier method and survival among groups was compared using the Log-Rank test. The prognostic value of variables was examined using a Cox-proportional hazard model. Multivariate analysis, considering all variables with a P-value < 0.10 in the univariate analysis, was performed to identify the independent predictors for adverse events. Probability was considered significant at a level of P ≤ 0.05. Analysis was performed using STATISTICA 6.1 (Statsoft Inc, Tulsa, Okla).
RESULTS
Characteristics of MI Cases
Table 1 shows the main baseline characteristics of the investigated MI patients. The NSTEMI group showed a higher percentage of cardiovascular risk factors compared with the STEMI group. As a contrary, current smokers are more frequent in the STEMI group. The incidence of previous MI and coronary revascularization (percutaneous or surgical) was higher in the NSTEMI group. Overall, 12.5% of patients had Killip class > 1 at entry (14.4% in STEMI vs 6.9% in NSTEMI; P = 0.04). The genotype distributions of FXIIIA-V34L and FXIIIB-H95R were as follows: VV, n = 350 (62.5%); VL, n = 182 (32.5%); LL, n = 28 (5.0%) and HH, n = 461 (82.3%); HR, n = 91 (16.3%); RR, n = 8 (1.4%) respectively. There were no significant differences between STEMI and NSTEMI patients both for FXIIIA and FXIIIB polymorphisms distributions (data not shown) as well as for mono-vessel and multi-vessel disease.
Clinical Outcome
After one-year follow-up, we observed an overall number of 137 adverse events (24.5%) of which 67 were heart failures (12.0%), 44 deaths (7.9%), and 26 reinfarctions (4.6%). Among these, 99 events were in the STEMI-group and 38 in the NSTEMI-group (23.8% vs 26.4%, P = 0.61). Cases satisfying the combined endpoint had older mean age (68 ± 11 vs 66 ± 12, P = 0.04), more often had previous MI (25% vs 16%, P = 0.015) and displayed higher Killip class at entry (17% vs 11%, P = 0.008).
Table 2 compares the different endpoints in the different sub-groups of MI patients stratified by FXIIIA genotypes and shows the respective risk values referred to the VV34-genotype. It notes an underrepresentation of adverse events in the L34-carriers for all subsets of cases investigated and different endpoints considered. The statistical significance was practically reached in all the MI-subgroups analyzed and for the different endpoints considered with the exception of reinfarction, which never yielded significance. In the NSTEMI group, significance was not obtained for large part of the endpoints considered, also because of the small number of cases included.
Table 2.
Different endpoints at one-year of follow-up in the whole cohort and in the sub-groups of cases stratified by FXIIIA genotype
| Adverse cardiovascular events (1-year follow-up)
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
MI-group |
aWhole (n = 560)
|
bSTEMI (n = 416)
|
cp-PCI (n = 374)
|
do-PCI (n = 506)
|
eNSTEMI (n = 144)
|
||||||
| FXIII-genotype | VV34 (n = 350) | L34 (n = 210) | VV34 (n = 257) | L34 (n = 159) | VV34 (n = 228) | L34 (n = 146) | VV34 (n = 315) | L34 (n = 191) | VV34 (n = 93) | L34 (n = 51) | |
| Death | 34 (9.7) | 10 (4.7) | 27 (10.5) | 7 (4.4) | 21 (9.2) | 6 (4.1) | 30 (9.5) | 10 (5.2) | 7 (7.5) | 3 (5.8) | |
| OR, CI, P | 2.38 (1.12–5.0) | 0.024 | 2.94 (1.3–7.1) | 0.019 | 2.85 (1.05–7.7) | 0.039 | 2.1 (1.1–4.54) | 0.041 | 1.31 (0.35–5.5) | NS | |
| fre-MI | 21 (6.0) | 5 (2.4) | 12 (4.7) | 4 (2.5) | 10 (4.4) | 4 (2.7) | 17 (5.4) | 5 (2.6) | 9 (9.7) | 1 (2.0) | |
| OR, CI, P | 2.57 (0.97–6.8) | 0.065 | 1.88 (0.60–5.9) | NS | 1.64 (0.50–5.1) | NS | 2.1 (0.8–5.7) | NS | 5.2 (0.64–41.6) | NS | |
| gHF | 46 (13.1) | 21 (10) | 33 (12.8) | 16 (10) | 30 (13) | 15 (10.2) | 43 (13.6) | 19 (10) | 13 (14.0) | 5 (9.8) | |
| hOR, CI, P | 1.72 (1.07–2.6) | 0.017 | 1.72 (1.03–2.94) | 0.039 | 1.72 (1.0–3.03) | 0.051 | 1.71 (1.06–2.7) | 0.027 | 1.59 (0.7–3.8) | NS | |
| Composite EP | 101 (28.8) | 36 (17.1) | 72 (28) | 27 (16.9) | 61 (26.8) | 25 (17.1) | 90 (28.6) | 34 (17.8) | 29 (31.2) | 9 (17.6) | |
| OR, CI, P | 2.0 (1.35–3.0) | 0.0004 | 1.92 (1.25–3.2) | 0.0038 | 1.81 (1.13–3.0) | 0.014 | 1.88 (1.25–2.9) | 0.0022 | 2.2 (1.09–4.5) | 0.037 | |
Whole, all MI cases.
STEMI, patients showing ST-segment elevation MI at enrollment.
p-PCI, STEMI cases eligible for primary PCI.
o-PCI, overall PCI, all cases eligible for primary or elective PCI.
NSTEMI, patients not showing ST-segment elevation MI at enrollment.
re-MI, indicates reinfarction.
HF, indicates heart failure.
ORs-values were calculated comparing the specific adverse cardiac events found in the VV34- versus those in the L34-carrier group for each category of patients, and the risk values are referred to the VV34-genotype.
Number and percentage are referred to and calculated within specific genotype and specific MI-group.
P-values above 0.10 are not shown.
NS, indicates not statistically significant results.
In regard to the overall number of adverse events after 30 days follow-up, we recorded a total of 54 (9.6%) of which 26 were heart failure (4.64%), 21 deaths (3.75%) and seven reinfarctions (1.25%). As for the longer follow-up, the number of adverse events at 30 days was under-represented in the L34-carriers respect to VV34-genotype (22.2% vs 77.8%; P = 0.022). Similar ratios were obtained in all the groups of patients considered (data not shown).
Conversely, FXIIIB polymorphism did not yield any significant result in any group for both follow-up monitored (data not shown).
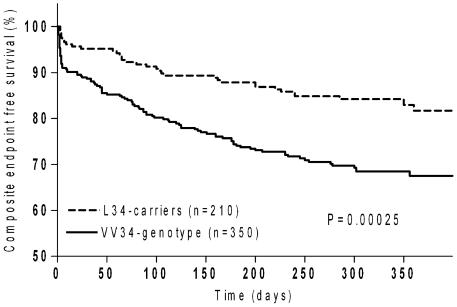
The Kaplan-Meier survival analysis at one-year showed significant differences in reaching the combined endpoint when stratified by FXIIIA genotypes in the whole group of cases (log-rank test, P = 0.00025) as well as in STEMI, NSTEMI, primary-PCI and overall-PCI-groups (log-rank tests, P = 0.001, P = 0.025, P = 0.009 and P = 0.001 respectively). Figure 1 shows different survival rates in the whole group of cases being significantly increased among L34-carriers. Accordingly, L34-carriers had about two-times lower probability to develop adverse events when compared with VV34-genotype (HR = 0.49; 0.34–0.74).
Figure 1.
Composite endpoint free survival in the case group according to FXIIIA V34L polymorphism. P = 0.00025, at log-rank test. HRs was 0.49 (95% CI, 0.34–0.74) for FXIIIA L34-carriers vs VV34-carriers.
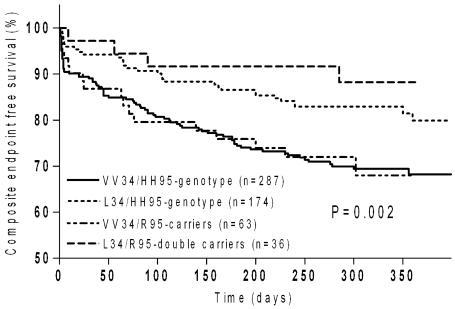
No significant survival differences were detectable in any of the subgroups considered when patients were stratified according to H95R genotypes (data not shown). Interestingly, among the L34-patients, those carrying in combination the R95-allele had lower probability to experience adverse events at one-year (Figure 2) and neither death nor reinfarction were recorded. This could be due to a fortuitous overrepresentation of L34-homozygotes among L34/R95-double carriers or it could be by chance, or one could suppose a synergistic protective interaction between the two polymorphic alleles. L34-homozygotes were equally distributed between the two subgroups (P = 0.90), but the log-rank test comparing survival between L34-carriers with and without the R95-allele did not yield significance (HR = 0.78, 95% CI, 0.45–1.2; P = 0.35). The very low number of cases carrying both substitutions could, in part, account for this finding.
Figure 2.
Composite endpoint free survival in the case groups according to both FXIII polymorphisms. Whole log-rank test, P = 0.002. HR = 0.78 (95% CI, 0.45–1.2) : L34/R95-double carriers (– – –) versus L34/HH95-genotype (- - -); (P = 0.35). HR = 0.6 (95% CI, 0.44-0.9) : L34/R95-double carriers (– – –) versus VV34/HH95-double homozygotes (—); (P = 0.019). HR = 0.49 (95% CI, 0.3-0.8) : L34/HH95-genotype (- - -) versus VV34/HH95-double homozygotes (—); (P = 0.002).
As for the longer follow-up, Kaplan-Meier analysis at 30 days showed different survival rates in the whole group of patients being significantly increased among L34-carriers (log-rank test, P = 0.009) (data not shown). Other sub analyses at 30-day follow-up were not assessed due to the shortage of events in the subgroups analyzed.
Summarizing, the worst clinical outcome was reserved to the VV34-homozygotes in both 30-day and one-year follow-up irrespective to the coexistence, or lack of coexistence, of the R95-allele. Indeed, at one-year, comparing HH95-homozygotes with versus those without L34-allele, the log-rank test was significant (HR = 0.49; 95% CI, 0.3–0.8; P = 0.002) (Figure 2).
Prognostic Value of Variables
In the Cox proportional hazards regression, age, previous MI, and FXIIIA-V34L variant were significant predictors of the combined endpoint at one-year follow-up. Killip class reached a borderline P-values (P = 0.09). HRs for these parameters are reported in Table 3. By incorporating as putative predictors all significant variables at univariate analysis plus the selected arterial risk factors and vessel disease, the independent predictors of composite endpoint were age, previous MI and FXIIIA-V34L variant.
Table 3.
Cox proportional hazards regression tests for predictors of combined end-point in the whole group of patients
| Variables | Hazard Ratios (95%CI) (1-year follow-up) | P-value |
|---|---|---|
| Univariate Analysis | ||
| aAge (years) | 1.7 (1.2 – 2.3) | 0.02 |
| bKillip class > 1 | 1.4 (0.9 – 2.1) | 0.09 |
| cPrevious MI | 1.6 (1.1 – 2.4) | 0.01 |
| dFXIIIA VV-homozygotes | 2.2 (1.3 – 2.9) | 0.004 |
| Multivariate Analysis | ||
| Age (years) | 1.7 (1.2 – 2.4) | 0.04 |
| Previous MI | 1.6 (1.2 – 2.4) | 0.02 |
| FXIIIA VV-homozygotes | 2.0 (1.4 – 3.0) | 0.005 |
Age was analyzed as below versus above the mean value (66.5 years).
Killip class as 1 versus ≥ 2.
previous MI as yes versus no.
FXIIIA gene variant as VV34-homozygote versus L34-carrier.
Bleeding Complications According to FXIII Genotypes
Table 4 shows a global frequency of bleeding complications of 8.6% (n = 48) in the whole group of cases. Major bleedings were 5 (0.9%) and minor bleedings were 43 (7.7%). The incidence of bleeding was higher in the L34-carriers than in VV34-homozygotes considering either minor bleeding or any (P = 0.0001 and P < 0.0001 respectively). Conversely, FXIIIB-H95R did not show any influence in bleeding complications nor did the coexistence of both variants (data not shown). It is to note that all the bleedings recorded were observed within the 1st 30 days of monitoring.
Table 4.
FXIII Polymorphisms and incidence of bleeding complications
| FXIII V34L
|
FXIII H95R
|
||||||
|---|---|---|---|---|---|---|---|
| Bleeding | Any genotype (n = 560) | VV (n = 350) | VL + LL (n = 210) | P | HH (n = 461) | HR + RR (n = 99) | P |
| Major, (n, %) | 5 (0.9) | 2 (0.6) | 3 (1.4) | NS | 4 (0.87) | 1 (1.0) | NS |
| Minor, (n, %) | 43 (7.7) | 16 (4.6) | 27 (12.9) | 0.0001 | 34 (7.4) | 9 (9.1) | NS |
| Any bleeding, (n, %) | 48 (8.6) | 18 (5.1) | 30 (14.3) | < 0.0001 | 38 (8.3) | 10 (10.1) | NS |
FXIII Levels after MI
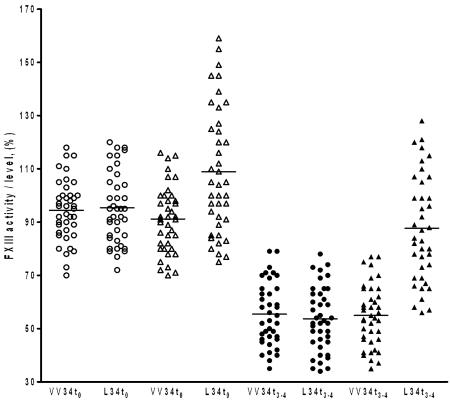
FXIII levels were assessed in a subgroup of 80 MI cases (VV34-genotype, n = 40 and L-34-carriers, n = 40) at enrollment (t0) and over the 3rd-4th day (t3–4) post-infarction period. Mean FXIII antigen levels were homogeneously depressed regardless of the different genotype of patients (trend-test; P = 0.89) over the t3–4 post-infarction period (FXIII % whole group, 54.4 ± 6.5; normal range 65–120%) with a non-significant, slightly lower mean level in the L34-carriers (FXIII%, 55.0 ± 6.2 and 53.8 ± 6.7 respectively for VV34- and L34-group; P = 0.33). FXIII t3–4 levels were significantly lower (P < 0.001) also when compared with the respective baseline t0 values (FXIII% whole group, 95.0 ± 10.1; 94.5 ± 10.3, and 95.4 ± 9.9 respectively for VV34- and L34-group). This was in agreement with the reduction of FXIII reported in patients over the 0–10-day post-infarction period (37). Conversely, mean FXIII activity after MI resulted significantly higher in L34-carriers when compared with VV34-genotype (FXIII %, 89.5 ± 12.5 vs 55.1 ± 6.5; P < 0.001). L34-carriers, despite similar antigen reduction and because of higher baseline activity levels, kept practically normal FXIII activity after MI. Figure 3 shows in detail levels and activity distribution of FXIII according to different genotypes and time of assessment. (The L34-carrier group consisted of 30 heterozygotes and 10 homozygotes).
Figure 3.
FXIII antigen levels (circles) and activity (triangles) assessed in a subgroup of 80 MI patients (VV34, n = 40 and L34-carriers, n = 40) at enrollment (t0, blank symbols) and at 3rd–4th day post-myocardial infarction (t3–4, black symbols). The significant higher mean FXIII activity of the L34-carriers present at t0, was observed also 3–4 days after MI when compared with the respective group with VV34-genotype. On the contrary, FXIII antigen levels dropped off after MI independently from the FXIII genotype. No differences in antigen levels were instead observed between different genotypes at both t0 and t3–4 times. The horizontal lines indicate FXIII mean values.
DISCUSSION
We investigated the effects of two common FXIII gene variants (i.e. FXIIIA-V34L and FXIIIB-H95R) on survival in a cohort of 560 cases after acute MI. The main finding of our survey is the identification of an independent molecular prognostic factor (i.e. FXIIIA L34-allele) against the occurrence of future adverse cardiac events in patients after acute MI with no effects on major bleeding complications. Globally, survival probability improved about two-fold for L34-carriers in all the groups of patients analyzed and for the different endpoints considered. Increased survival probability also remained in multivariate analyses at one-year follow-up.
Fibrin fibers, cross-linked by FXIIIA L34-variant, are thinner and have altered permeation characteristics which may influence thrombosis risk (38). To date, it has been hypothesized that fibrinogen levels (38), the early and wasteful activation of FXIII L34-zymogen, with consequent premature depletion from the circulation (39), and its lesser reliance on fibrin during clot formation (40), might be responsible for the cardio-protective effects of the L34-allele. As the FXIIIB-H95R variant is concerned, published data on the association with MI or thrombotic disease are very few and more controversial, yielding protection against MI (29,30) and risk for venous thrombosis in similar setting of populations (28). FXIIIB-H95R is associated with increased dissociation rate of the A2B2 tetramer following activation by thrombin. Because R95-allele possesses increased subunit dissociation independently from L34-allele (28), the two variants may have additive effects on FXIII activation. Thus, a synergistic interaction might account for effects on MI occurrence referring to the hypotheses mentioned above (38–40). In our survey, we didn’t find any significant effects on survival by R95-allele by itself or in combination with L34-allele, reserving the worst clinical outcome mainly to the VV34-genotype irrespective to the coexistence of the R95-allele. A high number of studies investigated V34L-variant and MI risk (20–27), few studies reported data on H95R-variant and thrombosis (28–30), while no studies exist on long follow-up after MI for such FXIII gene variants.
Studies aimed to investigate molecular genetic factors predicting restenosis after PCI, or associated with adverse cardiac events, suggested that common polymorphisms may affect clinical outcome and survival and/or interact pharmacogenetically with treatment (41–43). Conversely, a recent paper investigating on the classical thrombophilic molecular markers and the occurrence of adverse cardiac events after successful coronary stenting did not reveal consistent associations (14). In particular, no data are available on FXIII gene variants and the efficacy of mechanical reperfusion or on survival in patients after acute MI, but two studies on the efficacy of pharmacological reperfusion, ascribed to FXIIIA L34-allele, show a reduced efficacy of thrombolysis and the worst outcome at 24h (15,16). No data on longer follow-up and/or considering diverse endpoints are available. We found that the overall survival rate after acute MI was positively influenced by L34-allele, imputing to the VV34-genotype the worst clinical outcome. The latter is considered an independent negative predictor of the combined endpoint assessed as heart failure, re-infarction or cardiovascular death at both 30-day and one-year follow-up.
When myocardial infarction occurs, the future clinical outcome (i.e. survival) is strictly associated with infarction lesion extension and myocardial healing (44). This could in part explain why, in sub-analyses for single endpoints, re-infarction never reached statistical significance. A lower reinfarction rate would either be caused by slower lesion formation or reduced thrombogenic status. Thus, reinfarction endpoint could not completely fit with a healing hypothesis. On the other hand, the purely clinical assessment of heart failure entails limitations. In this view, it would be highly desirable to influence healing of the cardiac wound to maintain structure and function of the heart and to improve survival (44).
An attempt to explain the positive association of L34-allele with survival (protective effect) comes from its higher associated FXIII activity. We found that despite of the significant FXIII antigen reductions observed after MI apart from different FXIII genotypes, L34-carriers kept higher FXIII activity (P < 0.001).
All this could explain in part why a variant at higher activity is found protective for cardiovascular disease, and is in line with the reported association between low FXIII levels and decreased survival after MI in the presence of moderate FXIII reductions in a mouse model (1). The latter combined with impaired myocardial wound healing, remodeling, and unrestrained MMP-9 levels. Normal wound healing implies deposition of numerous ECM components, fibrin and collagen included, responsible for a mechanically stable scar. In addition, angiogenesis, fibroblast proliferation, collagen synthesis and its cross-linking with fibrin fibers are pivotal processes in wound healing and they are all regulated by FXIII molecule (1–6,17,18,45). We recently reported FXIII contrasted MMPs detrimental effects in human dermal fibroblast cultured cells while also improving cell proliferation (46). In addition, chronic skin lesions were negatively associated with FXIII activity and with L34-allele (7). Finally, other FXIII gene variants responsible for increased molecule activity were associated with better prognosis and shorter healing time after surgery (8,47). Considering that VV34-carriers have a basal mean FXIII activity of about 70%–90% (7,33,this paper) and that after acute MI, a reduction of circulating FXIII begins (37,this paper), VV34-patients might be disadvantaged in recovering myocardial functions respective to L34-carriers with significantly higher activity (7,33,this paper). This is in line with the fact that moderate low levels of FXIII (~70%) also experienced fatal outcome after MI and that FXIII-injected mice significantly improved survival (1).
In conclusion, different healing processes, genetically determined, might also be hypothesized in cases after myocardial injury. Further larger clinical investigations or multicenter studies, and other polymorphic gene variants analyses are needed to understand whether or not FXIII genotyping and level monitoring may be useful prognostic markers to select cases with poor clinical outcome after cardiovascular events. FXIII positive effects could pave the way to utilize FXIII molecule as a tailored treatment (1,48) to improve myocardial healing, recovery of functions, and survival after infarction.
Acknowledgments
This study was supported in part by the Italian MIUR funds and by a grant from Fondazione Cassa di Risparmio di Cento, Italy.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Nahrendorf M, et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation. 2006;113:1196–202. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dardik R, et al. Evaluation of the pro-angiogenic effect of factor XIII in heterotopic mouse heart allografts and FXIII-deficient mice. Thromb Haemost. 2006;95:546–50. doi: 10.1160/TH05-06-0409. [DOI] [PubMed] [Google Scholar]
- 3.Dardik R, et al. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler Thromb Vasc Biol. 2003;23:1472–7. doi: 10.1161/01.ATV.0000081636.25235.C6. [DOI] [PubMed] [Google Scholar]
- 4.Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost. 2006;1:19–25. doi: 10.1111/j.1538-7836.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- 5.Herouy Y, Hellstern MO, Vanscheidt W, Schopf E, Norgauer J. Factor XIII-mediated inhibition of fibrinolysis and venous leg ulcers. Lancet. 2000;355:1970–1. doi: 10.1016/S0140-6736(00)02333-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown LF, et al. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142:273–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Gemmati D, et al. Factor XIII V34L polymorphism modulates the risk of chronic venous leg ulcer progression and extension. Wound Repair Regen. 2004;12:512–7. doi: 10.1111/j.1067-1927.2004.012503.x. [DOI] [PubMed] [Google Scholar]
- 8.Gemmati D, et al. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J Vasc Surg. 2006;44:554–62. doi: 10.1016/j.jvs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Keeley EC, Grines CL. Primary percutaneous coronary intervention for every patient with ST segment elevation myocardial infarction: what stands in the way? Ann Intern Med. 2004;141:298–304. doi: 10.7326/0003-4819-141-4-200408170-00010. [DOI] [PubMed] [Google Scholar]
- 10.Radke PW, Kaiser A, Frost C, Sigwart U. Outcome after treatment of coronary instent restenosis; results from a systematic review using meta-analysis techniques. Eur Heart J. 2003;24:266–73. doi: 10.1016/s0195-668x(02)00202-6. [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, et al. Clinical and angiographic follow-up after primary stenting in acute myocardial infarction: the Primary Angioplasty in Myocardial Infarction (PAMI) stent pilot trial. Circulation. 1999;99:1548–54. doi: 10.1161/01.cir.99.12.1548. [DOI] [PubMed] [Google Scholar]
- 12.Heggunje PS, et al. Procedural success versus clinical risk status in determining discharge of patients after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1400–7. doi: 10.1016/j.jacc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 13.Magid DJ, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA. 2005;294:803–12. doi: 10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 14.Marcucci R, et al. PAI-1 and homocysteine, but not lipoprotein (a) and thrombophilic polymorphisms, are independently associated with the occurrence of major adverse cardiac events after successful coronary stenting. Heart. 2006;92:337–81. doi: 10.1136/hrt.2005.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roldan V, Corral J, Marin F, Rivera J, Vicente V. Effect of factor XIII Val34Leu polymorphism on thrombolytic therapy in premature myocardial infarction. Thromb Haemost. 2002;88:354–5. [PubMed] [Google Scholar]
- 16.Marin F, et al. A pharmacogenetic effect of factor XIII valine 34 leucine polymorphism on fibrinolytic therapy for acute myocardial infarction. J Am Coll Cardiol. 2005;45:25–9. doi: 10.1016/j.jacc.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost. 2001;86:57–65. [PubMed] [Google Scholar]
- 18.Bereczky Z, Katona E, Muszbek L. –2004) Fibrin stabilization (factor XIII), fibrin structure and thrombosis. Pathophysiol Haemost Thromb. 2003;33:430–7. doi: 10.1159/000083841. [DOI] [PubMed] [Google Scholar]
- 19.Fatah K, et al. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost. 1996;76:535–40. [PubMed] [Google Scholar]
- 20.Kohler HP, Ariens RA, Whitaker P, Grant PJ. A common coding polymorphism in the FXIII A-subunit gene (FXIII Val34Leu) affects cross-linking activity. Thromb Haemost. 1998;80:704. [PubMed] [Google Scholar]
- 21.Ariens RA, et al. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood. 2000;96:988–95. [PubMed] [Google Scholar]
- 22.Kohler HP, et al. Association of a common polymorphism in the factor XIII gene with myocardial infarction. Thromb Haemost. 1998;79:8–13. [PubMed] [Google Scholar]
- 23.Wartiovaara U, et al. Association of FXIII Val34Leu with decreased risk of myocardial infarction in Finnish males. Atherosclerosis. 1999;142:295–300. doi: 10.1016/s0021-9150(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 24.Gemmati D, et al. A common mutation in the gene for coagulation factor XIII-A (Val34Leu): a risk factor for primary intracerebral hemorrhage is protective against atherothrombotic diseases. Am J Hematol. 2001;67:183–8. doi: 10.1002/ajh.1104. [DOI] [PubMed] [Google Scholar]
- 25.Martini CH, Doggen CJ, Cavallini C, Rosendaal FR, Mannucci PM. No effect of polymorphisms in prothrombotic genes on the risk of myocardial infarction in young adults without cardiovascular risk factors. J Thromb Haemost. 2005;3:177–9. doi: 10.1111/j.1538-7836.2004.01080.x. [DOI] [PubMed] [Google Scholar]
- 26.Atherosclerosis, Thrombosis, and Vascular Biology Italian Study Group. No evidence of association between prothrombotic gene polymorphisms and the development of acute myocardial infarction at a young age. Circulation. 2003;107:1117–22. doi: 10.1161/01.cir.0000051465.94572.d0. [DOI] [PubMed] [Google Scholar]
- 27.Warner D, Mansfield MW, Grant PJ. Coagulation factor XIII and cardiovascular disease in UK Asian patients undergoing coronary angiography. Thromb Haemost. 2001;85:408–11. [PubMed] [Google Scholar]
- 28.Komanasin N, et al. A novel polymorphism in the factor XIII B-subunit (His95Arg): relationship to subunit dissociation and venous thrombosis. J Thromb Haemost. 2005;3:2487–96. doi: 10.1111/j.1538-7836.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- 29.Reiner AP, et al. Genetic variants of coagulation factor XIII, postmenopausal estrogen therapy, and risk of nonfatal myocardial infarction. Blood. 2003;102:25–30. doi: 10.1182/blood-2002-07-2308. [DOI] [PubMed] [Google Scholar]
- 30.Doggen CJ, Reiner AP, Vos HL, Rosendaal FR. Two factor XIII gene polymorphisms associated with a structural and functional defect and the risk of myocardial infarction in men. J Thromb Haemost. 2003;1:2056–8. doi: 10.1046/j.1538-7836.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 31.Anwar R, et al. Genotype/phenotype correlations for coagulation factor XIII: specific normal polymorphisms are associated with high or low factor XIII specific activity. Blood. 1999;93:897–905. [PubMed] [Google Scholar]
- 32.de Lange M, et al. Joint linkage and association of six single-nucleotide polymorphisms in the factor XIII-A subunit gene, point to V34L as the main functional locus. Arterioscler Thromb Vasc Biol. 2006;26:1914–9. doi: 10.1161/01.ATV.0000231538.60223.92. [DOI] [PubMed] [Google Scholar]
- 33.Kohler HP, Ariens RA, Whitaker P, Grant PJ. A common coding polymorphism in the FXIII A-subunit gene (FXIIIVal34Leu) affects cross-linking activity. Thromb Haemost. 1998;80:704. [PubMed] [Google Scholar]
- 34.American College of Cardiology/American Heart Association Task Force on Practice Guidelines. (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–75. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 35.Ballerini G, Gemmati D, Moratelli S, Morelli P, Serino ML. A photometric method for the dosage of factor XIII applied to the study of chronic hepatopathies. Thromb Res. 1995;78:451–6. doi: 10.1016/0049-3848(95)99611-b. [DOI] [PubMed] [Google Scholar]
- 36.Chew DP, Bhatt DL, Lincoff AM, Wolski K, Topol EJ. Clinical end point definitions following percutaneous coronary intervention and their relationship to late mortality: an assessment by attributable risk. Heart. 2006;92:945–50. doi: 10.1136/hrt.2005.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkjaersig N, Fletcher AP, Lewis M, Ittyerah R. Reduction of coagulation factor XIII concentration in patients with myocardial infarction, cerebral infarction, and other thromboembolic disorders. Thromb Haemost. 1977;38:863–73. [PubMed] [Google Scholar]
- 38.Lim BC, Ariens RA, Carter AM, Weisel JW, Grant PJ. Genetic regulation of fibrin structure and function: complex gene-environment interactions may modulate vascular risk. Lancet. 2003;361:1424–31. doi: 10.1016/S0140-6736(03)13135-2. [DOI] [PubMed] [Google Scholar]
- 39.Trumbo TA, Maurer MC. Examining thrombin hydrolysis of the factor XIII activation peptide segment leads to a proposal for explaining the cardioprotective effects observed with the factor XIII V34L mutation. J Biol Chem. 2000;275:20627–31. doi: 10.1074/jbc.M000209200. [DOI] [PubMed] [Google Scholar]
- 40.Maurer MC, Trumbo TA, Isetti G, Turner BT., Jr Probing interactions between the coagulants thrombin, Factor XIII, and fibrin(ogen) Arch Biochem Biophys. 2006;445:36–45. doi: 10.1016/j.abb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Segev A, et al. Thr325Ile polymorphism of the TAFI gene is related to TAFI antigen plasma levels and angiographic restenosis after percutaneous coronary interventions. Thromb Res. 2004;114:137–41. doi: 10.1016/j.thromres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Botto N, et al. C677T polymorphism of the methylenetetrahydrofolate reductase gene is a risk factor of adverse events after coronary revascularization. Int J Cardiol. 2004;96:341–5. doi: 10.1016/j.ijcard.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Toyofyuku M, et al. Influence of angiotensinogen M253T gene polymorphism and an angiotensin converting enzyme inhibitor on restenosis after percutaneous coronary intervention. Atherosclerosis. 2002;160:339–44. doi: 10.1016/s0021-9150(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 44.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Inbal A, et al. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94:432–7. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 46.Zamboni P, et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc Endovascular Surg. 2004;38:431–8. doi: 10.1177/153857440403800506. [DOI] [PubMed] [Google Scholar]
- 47.Tognazzo S, et al. Prognostic role of factor XIII gene variants in chronic venous leg ulcer. J Vasc Surg. 2006;44:815–9. doi: 10.1016/j.jvs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Nahrendorf M, Weissleder R, Ertl G. Does FXIII deficiency impair wound healing after myocardial infarction? PLoS ONE. 2006;20;1:e48. doi: 10.1371/journal.pone.0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]