Abstract
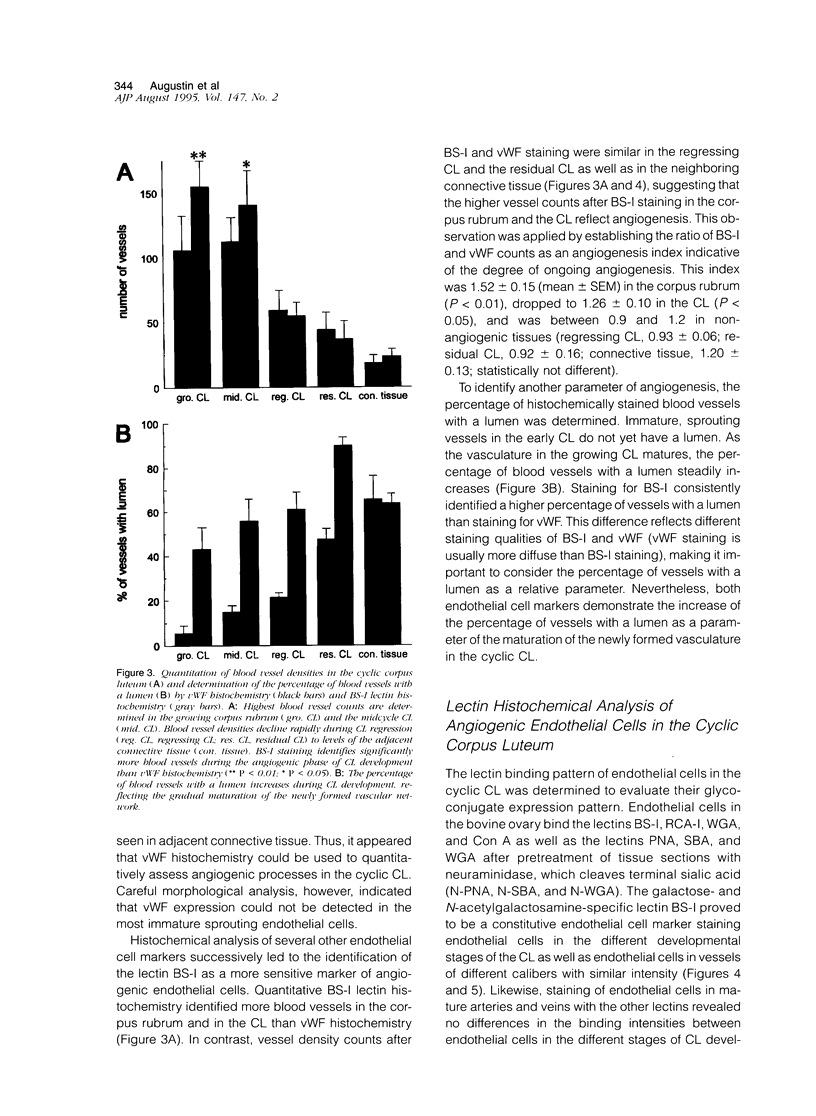
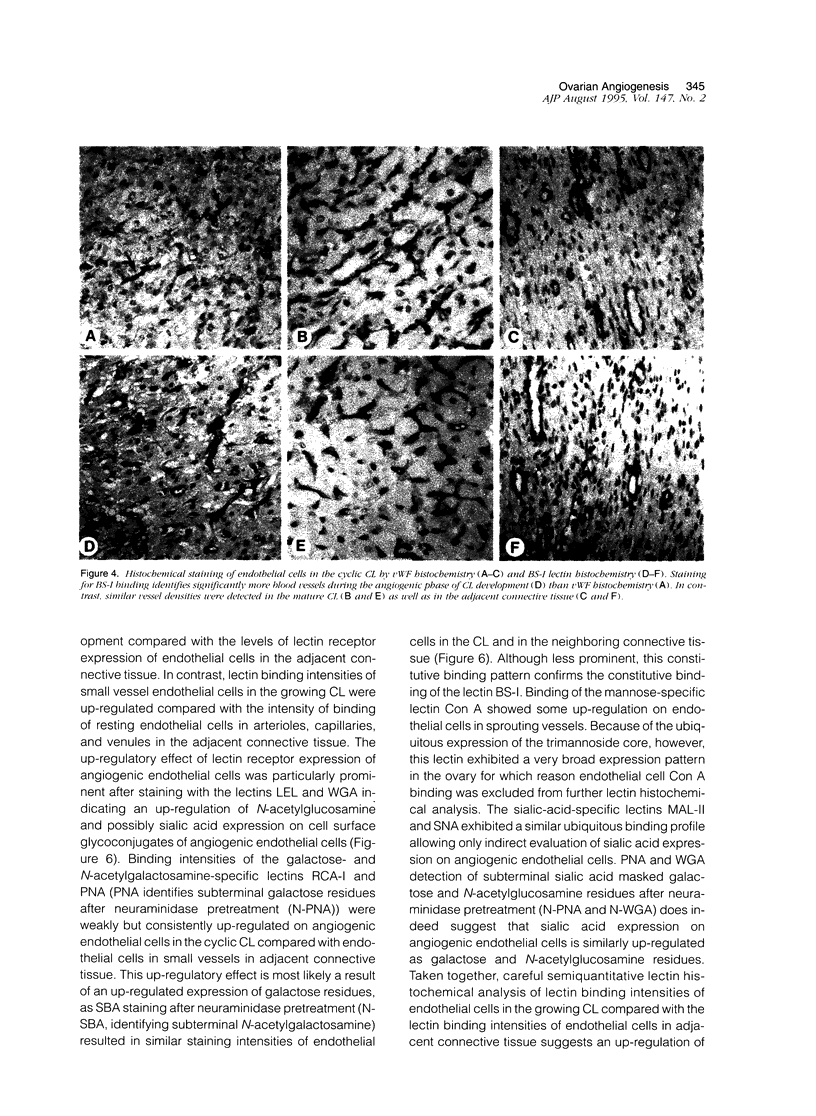
Angiogenesis occurs during embryogenesis and is a down-regulated process in the healthy adult that is almost exclusively linked to pathological conditions such as tumor growth, wound healing, and inflammation. Physiological angiogenic processes in the adult are restricted to the female reproductive system where they occur cyclically during the ovarian and uterine cycle as well as during pregnancy. By systematically analyzing the phenotypic changes of endothelial cells during bovine corpus luteum (CL) formation and regression, we have established a physiological model of blood vessel growth and regression. Quantitation of vessel density, percentage of vessels with lumen, and ratio of Bandeiraea simplicifolia-I to von Willebrand Factor-positive endothelial cells were established as parameters of angiogenesis. Sprouting endothelial cells invade the growing CL and continue to grow throughout the first third of the ovarian cycle. Thereafter the mature CL is characterized by a dense network of vessels with gradually decreasing vessel density. During luteolysis and for several weeks thereafter (regressing and residual CL) all newly formed vessels regress, which is accompanied by gradual foreshortening and rounding of endothelial cells and subsequent detachment. Based on histochemical detection of nucleosomal fragmentation products physiological blood vessel regression in the cyclic CL does not appear to involve endothelial cell apoptosis. Lectin histochemical analysis revealed a distinct alteration of endothelial cell glycoconjugate expression during ovarian angiogenesis comparable with the distinct pattern of hyperglycosylation of cultured migrating endothelial cells (up-regulation of binding sites for Lycopersicon esculentum lectin, wheat germ agglutinin, neuraminidase-treated peanut agglutinin, and Ricinus communis agglutinin-I on sprouting ECs). Northern blot analysis of glycosyltransferases during the different stages of angiogenesis revealed an up-regulation of beta-galactoside alpha 2,6-sialyltransferase and alpha 1,3-galactosyltransferase mRNA expression during the angiogenic stages of CL formation. These data establish the ovarian angiogenesis model as a suitable experimental system to study the functional and phenotypic properties of endothelial cells in sprouting and regressing blood vessels and provide additional evidence for the importance of endothelial cell surface glycoconjugates during angiogenesis.
Full text
PDF





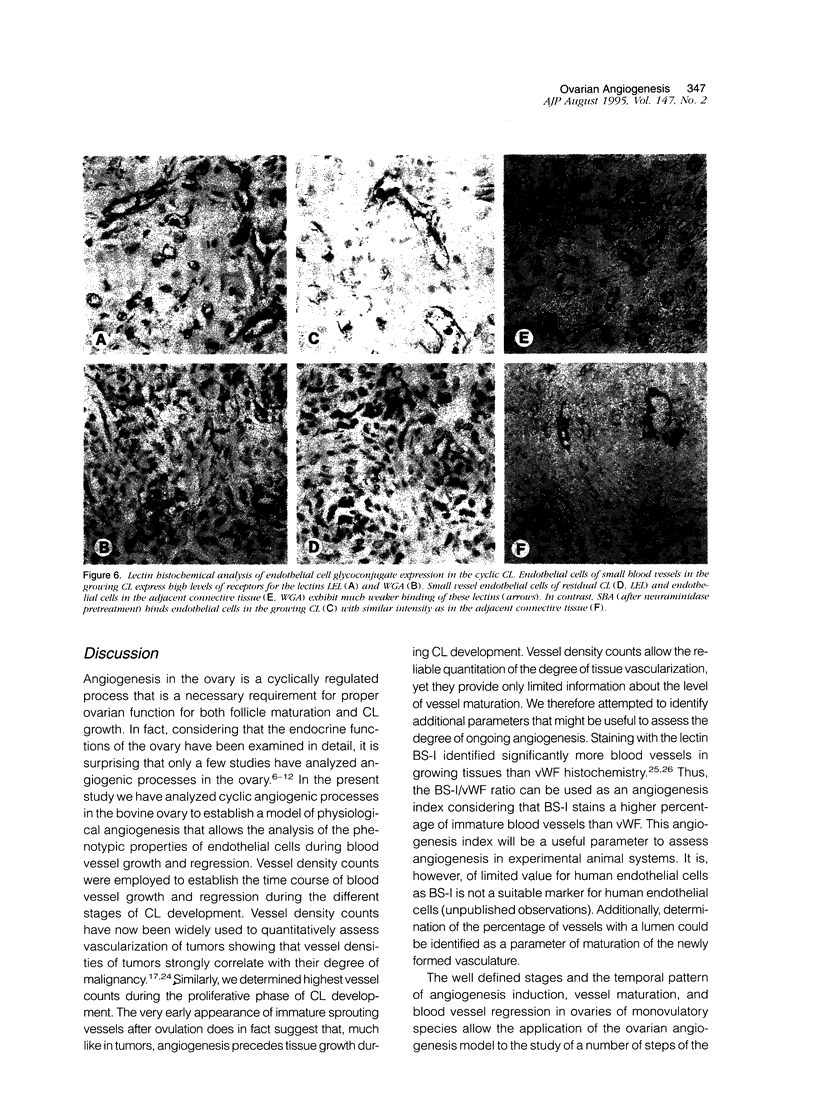
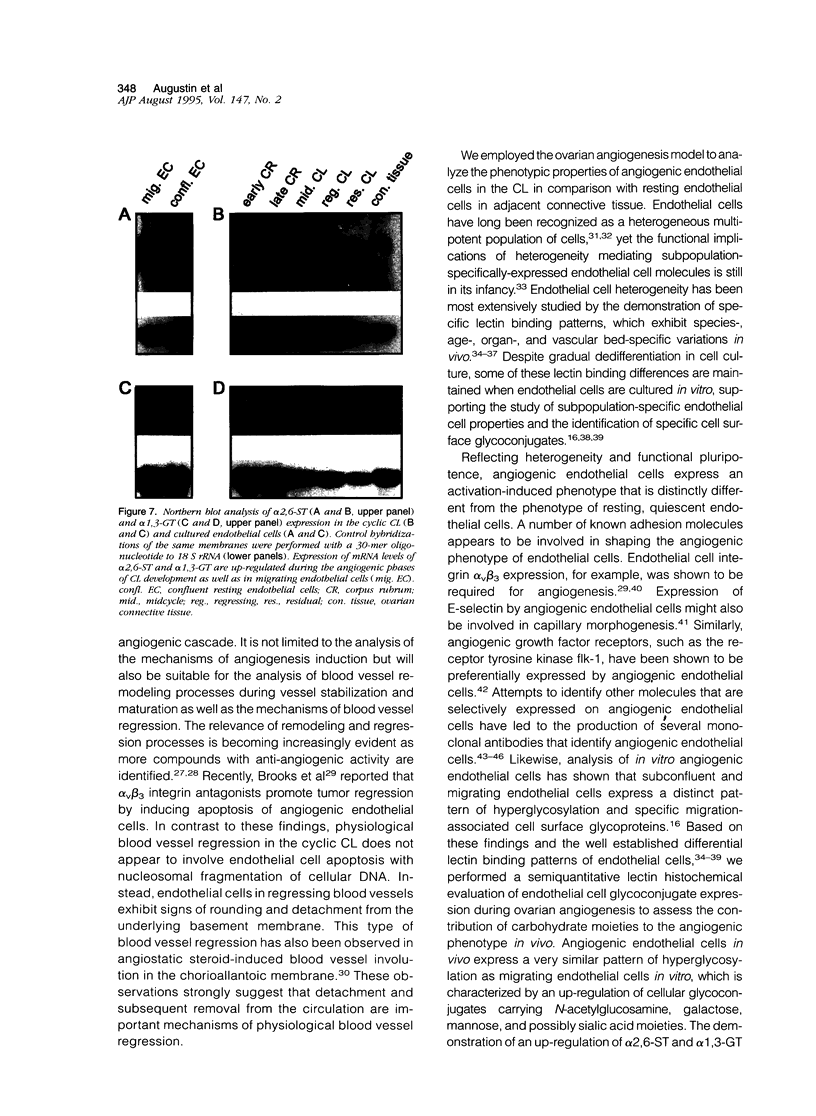
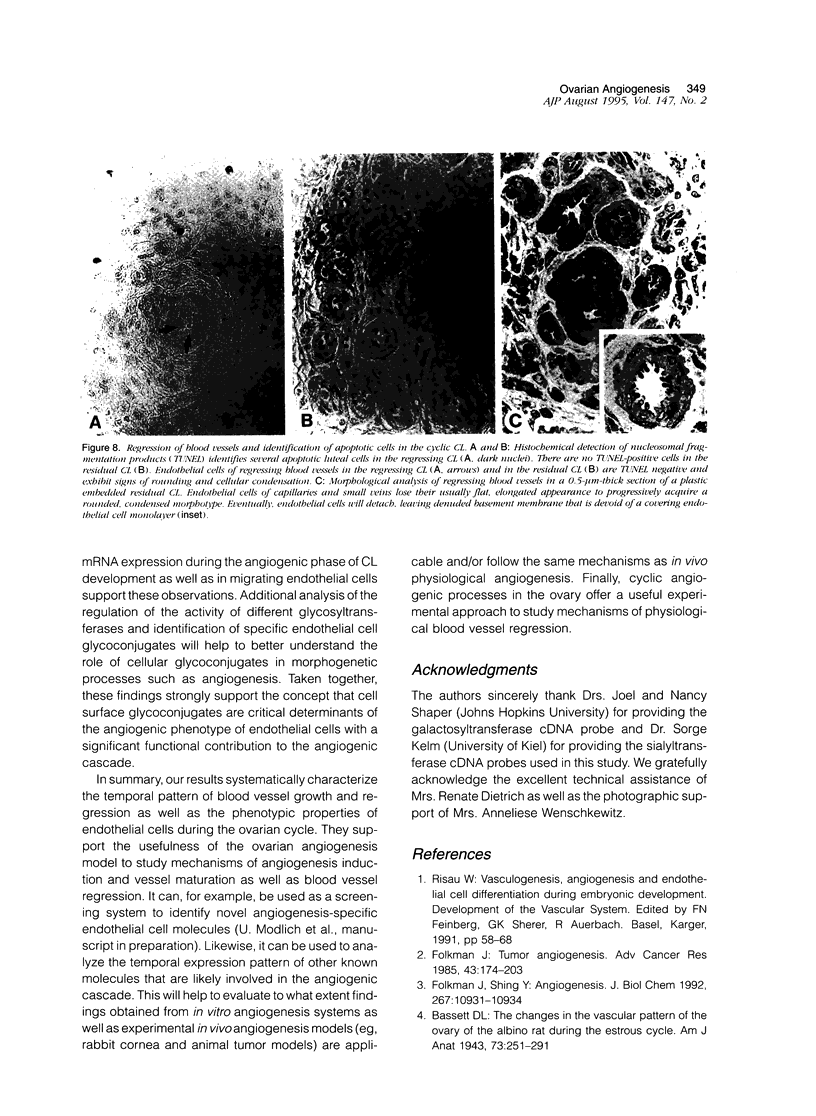


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Auerbach W., Polakowski I. Assays for angiogenesis: a review. Pharmacol Ther. 1991;51(1):1–11. doi: 10.1016/0163-7258(91)90038-n. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Vascular endothelial cell differentiation: organ-specificity and selective affinities as the basis for developing anti-cancer strategies. Int J Radiat Biol. 1991 Jul-Aug;60(1-2):1–10. doi: 10.1080/09553009114551401. [DOI] [PubMed] [Google Scholar]
- Augustin-Voss H. G., Pauli B. U. Migrating endothelial cells are distinctly hyperglycosylated and express specific migration-associated cell surface glycoproteins. J Cell Biol. 1992 Oct;119(2):483–491. doi: 10.1083/jcb.119.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin-Voss H. G., Voss A. K., Pauli B. U. Senescence of aortic endothelial cells in culture: effects of basic fibroblast growth factor expression on cell phenotype, migration, and proliferation. J Cell Physiol. 1993 Nov;157(2):279–288. doi: 10.1002/jcp.1041570210. [DOI] [PubMed] [Google Scholar]
- Augustin H. G., Kozian D. H., Johnson R. C. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. Bioessays. 1994 Dec;16(12):901–906. doi: 10.1002/bies.950161208. [DOI] [PubMed] [Google Scholar]
- Belloni P. N., Nicolson G. L. Differential expression of cell surface glycoproteins on various organ-derived microvascular endothelia and endothelial cell cultures. J Cell Physiol. 1988 Sep;136(3):398–410. doi: 10.1002/jcp.1041360303. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994 Dec 30;79(7):1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Christie K. N., Thomson C. Bandeiraea simplicifolia lectin demonstrates significantly more capillaries in rat skeletal muscle than enzyme methods. J Histochem Cytochem. 1989 Aug;37(8):1303–1304. doi: 10.1177/37.8.2754256. [DOI] [PubMed] [Google Scholar]
- Christofori G., Hanahan D. Molecular dissection of multi-stage tumorigenesis in transgenic mice. Semin Cancer Biol. 1994 Feb;5(1):3–12. [PubMed] [Google Scholar]
- DiPietro L. A., Polverini P. J. Role of the macrophage in the positive and negative regulation of wound neovascularization. Behring Inst Mitt. 1993 Aug;(92):238–247. [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Folkman J., Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992 Apr;3(2):89–96. [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Thakral K. K. Production a corpus luteum angiogenic factor responsible for proliferation of capillaries and neovascularization of the corpus luteum. Proc Natl Acad Sci U S A. 1978 Feb;75(2):847–851. doi: 10.1073/pnas.75.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazul-Bilska A. T., Reynolds L. P., Slanger W. D., Redmer D. A. Production of heparin-binding angiogenic factor(s) by bovine corpora lutea during pregnancy. J Anim Sci. 1992 Jan;70(1):254–262. doi: 10.2527/1992.701254x. [DOI] [PubMed] [Google Scholar]
- Gumkowski F., Kaminska G., Kaminski M., Morrissey L. W., Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24(1-2):11–23. [PubMed] [Google Scholar]
- Hagemeier H. H., Vollmer E., Goerdt S., Schulze-Osthoff K., Sorg C. A monoclonal antibody reacting with endothelial cells of budding vessels in tumors and inflammatory tissues, and non-reactive with normal adult tissues. Int J Cancer. 1986 Oct 15;38(4):481–488. doi: 10.1002/ijc.2910380405. [DOI] [PubMed] [Google Scholar]
- Han R. N., Tanswell A. K., Post M. Ontogeny of reactivity to endothelial cell markers during development of the embryonic and fetal rat lung. Histol Histopathol. 1992 Oct;7(4):591–597. [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986 Oct;119(4):1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Van den Eijnden D. H., Van Tunen A. J., Shaper N. L. Bovine alpha 1----3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- Kamat B. R., Brown L. F., Manseau E. J., Senger D. R., Dvorak H. F. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995 Jan;146(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Nickoloff B. J., Holgersson J., Seed B., Haines G. K., Burrows J. C., Leibovich S. J. 4A11, a monoclonal antibody recognizing a novel antigen expressed on aberrant vascular endothelium. Upregulation in an in vivo model of contact dermatitis. Am J Pathol. 1994 Feb;144(2):244–259. [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Kuzu I., Gatter K. C., Bicknell R. Heterogeneity of the endothelial cell and its role in organ preference of tumour metastasis. Trends Pharmacol Sci. 1991 Dec;12(12):462–467. doi: 10.1016/0165-6147(91)90637-8. [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Mills A. N., Haworth S. G. Changes in lectin binding patterns in the developing pulmonary vasculature of the pig lung. J Pathol. 1986 Jul;149(3):191–199. doi: 10.1002/path.1711490305. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Ferrara N., Schweigerer L., Mitchell R., Gospodarowicz D. Bovine granulosa cells produce basic fibroblast growth factor. Endocrinology. 1987 Aug;121(2):597–603. doi: 10.1210/endo-121-2-597. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Strubel N. A., Bischoff J. A role for sialyl Lewis-X/A glycoconjugates in capillary morphogenesis. Nature. 1993 Sep 16;365(6443):267–269. doi: 10.1038/365267a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M. Organ-related differences in binding of Dolichos biflorus agglutinin to vascular endothelium. Dev Biol. 1983 Apr;96(2):535–541. doi: 10.1016/0012-1606(83)90191-4. [DOI] [PubMed] [Google Scholar]
- Ravindranath N., Little-Ihrig L., Phillips H. S., Ferrara N., Zeleznik A. J. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992 Jul;131(1):254–260. doi: 10.1210/endo.131.1.1612003. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P., Healey J. H., Su S. L., Jaffe E. A., Old L. J. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L. P., Killilea S. D., Redmer D. A. Angiogenesis in the female reproductive system. FASEB J. 1992 Feb 1;6(3):886–892. [PubMed] [Google Scholar]
- Sasaki K., Watanabe E., Kawashima K., Sekine S., Dohi T., Oshima M., Hanai N., Nishi T., Hasegawa M. Expression cloning of a novel Gal beta (1-3/1-4) GlcNAc alpha 2,3-sialyltransferase using lectin resistance selection. J Biol Chem. 1993 Oct 25;268(30):22782–22787. [PubMed] [Google Scholar]
- Shweiki D., Itin A., Neufeld G., Gitay-Goren H., Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993 May;91(5):2235–2243. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda R., Tavassoli M. Mapping of the bone marrow sinus endothelium with lectins and glycosylated ferritins: identification of differentiated microdomains and their functional significance. J Ultrastruct Res. 1983 Sep;84(3):299–310. doi: 10.1016/s0022-5320(83)80009-4. [DOI] [PubMed] [Google Scholar]
- Stirling D., Waterman M. R., Simpson E. R. Expression of mRNA encoding basic fibroblast growth factor (bFGF) in bovine corpora lutea and cultured luteal cells. J Reprod Fertil. 1991 Jan;91(1):1–8. doi: 10.1530/jrf.0.0910001. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Kumar S., Pye D., van Agthoven A. J., Krupinski J., Hunter R. D. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993 May 28;54(3):363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- Weidner N., Carroll P. R., Flax J., Blumenfeld W., Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Wen D. X., Livingston B. D., Medzihradszky K. F., Kelm S., Burlingame A. L., Paulson J. C. Primary structure of Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase determined by mass spectrometry sequence analysis and molecular cloning. Evidence for a protein motif in the sialyltransferase gene family. J Biol Chem. 1992 Oct 15;267(29):21011–21019. [PubMed] [Google Scholar]