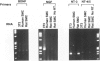Abstract
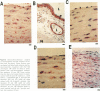
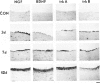
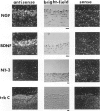
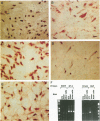
The neurotrophins, a family of related polypeptide growth factors including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin (NT)-3 and NT-4/5 promote the survival and differentiation of distinctive sets of embryonic neurons. Here we define a new functional role for neurotrophins, as autocrine or local paracrine mediators of vascular smooth muscle cell migration. We have identified neurotrophins, and their cognate receptors, the trk tyrosine kinases, in human and rat vascular smooth muscle cells in vivo. In vitro, cultured human smooth muscle cells express BDNF; NT-3; and trk A, B, and C Similarly, rat smooth muscle cells expressed all three trk receptors as well as all four neurotrophins. Moreover, NGF induces cultured human smooth muscle cell migration at subnanomolar concentrations. In the rat aortic balloon deendothelialization model of vascular injury, the expression of NGF, BDNF, and their receptors trk A and trk B increased dramatically in the area of injury within 3 days and persisted during the formation of the neointima. In human coronary atherosclerotic lesions, BDNF, NT-3, and NT-4/5, and the trk B and trk C receptors could be demonstrated in smooth muscle cells. These findings suggest that neurotrophins play an important role in regulating the response of vascular smooth muscle cells to injury.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldino F., Jr, Chesselet M. F., Lewis M. E. High-resolution in situ hybridization histochemistry. Methods Enzymol. 1989;168:761–777. doi: 10.1016/0076-6879(89)68057-3. [DOI] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991 Nov;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bornfeldt K. E., Raines E. W., Nakano T., Graves L. M., Krebs E. G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994 Mar;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Tissue localization of nerve growth factor and nerve growth factor receptors. Curr Top Microbiol Immunol. 1991;165:55–70. doi: 10.1007/978-3-642-75747-1_4. [DOI] [PubMed] [Google Scholar]
- Brogi E., Winkles J. A., Underwood R., Clinton S. K., Alberts G. F., Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993 Nov;92(5):2408–2418. doi: 10.1172/JCI116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Clegg D. O., Large T. H., Bodary S. C., Reichardt L. F. Regulation of nerve growth factor mRNA levels in developing rat heart ventricle is not altered by sympathectomy. Dev Biol. 1989 Jul;134(1):30–37. doi: 10.1016/0012-1606(89)90075-4. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creedon D., Tuttle J. B. Nerve growth factor synthesis in vascular smooth muscle. Hypertension. 1991 Dec;18(6):730–741. doi: 10.1161/01.hyp.18.6.730. [DOI] [PubMed] [Google Scholar]
- Djakiew D., Delsite R., Pflug B., Wrathall J., Lynch J. H., Onoda M. Regulation of growth by a nerve growth factor-like protein which modulates paracrine interactions between a neoplastic epithelial cell line and stromal cells of the human prostate. Cancer Res. 1991 Jun 15;51(12):3304–3310. [PubMed] [Google Scholar]
- Donovan M. J., Hempstead B. L., Horvath C., Chao M. V., Schofield D. Immunohistochemical localization of Trk receptor protein in pediatric small round blue cell tumors. Am J Pathol. 1993 Dec;143(6):1560–1567. [PMC free article] [PubMed] [Google Scholar]
- Donovan M. J., Hempstead B., Huber L. J., Kaplan D., Tsoulfas P., Chao M., Parada L., Schofield D. Identification of the neurotrophin receptors p75 and trk in a series of Wilms' tumors. Am J Pathol. 1994 Oct;145(4):792–801. [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Friedman W. J., Ernfors P., Persson H. Transient and persistent expression of NT-3/HDNF mRNA in the rat brain during postnatal development. J Neurosci. 1991 Jun;11(6):1577–1584. doi: 10.1523/JNEUROSCI.11-06-01577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Haaparanta T., Neufeld G. Basic fibroblast growth factor: expression in cultured bovine vascular smooth muscle cells. Eur J Cell Biol. 1988 Apr;46(1):144–151. [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Birge R. B., Fajardo J. E., Glassman R., Mahadeo D., Kraemer R., Hanafusa H. Expression of the v-crk oncogene product in PC12 cells results in rapid differentiation by both nerve growth factor- and epidermal growth factor-dependent pathways. Mol Cell Biol. 1994 Mar;14(3):1964–1971. doi: 10.1128/mcb.14.3.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Rabin S. J., Kaplan L., Reid S., Parada L. F., Kaplan D. R. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992 Nov;9(5):883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Horvath C. M., Wolven A., Machadeo D., Huber J., Boter L., Benedetti M., Hempstead B., Chao M. V. Analysis of the trk NGF receptor tyrosine kinase using recombinant fusion proteins. J Cell Sci Suppl. 1993;17:223–228. doi: 10.1242/jcs.1993.supplement_17.31. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Ibáez C. F., Nye S. H., McClain J., Jones P. F., Gies D. R., Belluscio L., Le Beau M. M., Espinosa R., 3rd, Squinto S. P. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L., Reidy M. A. Basic fibroblast growth factor: its role in the control of smooth muscle cell migration. Am J Pathol. 1993 Oct;143(4):1024–1031. [PMC free article] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Kokaia Z., Bengzon J., Metsis M., Kokaia M., Persson H., Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Smeyne R. J., Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994 Jan;14(1):14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Expression of basic fibroblast growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries. An en face study. Circ Res. 1993 Sep;73(3):589–595. doi: 10.1161/01.res.73.3.589. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Borth W., Brayton C. F., Weksler B. B. Fucoidan is a non-anticoagulant inhibitor of intimal hyperplasia. Biochem Biophys Res Commun. 1992 Apr 30;184(2):773–781. doi: 10.1016/0006-291x(92)90657-7. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Shooter E. M. The nerve growth factor family of receptors. Trends Neurosci. 1992 Sep;15(9):323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Middlemas D. S., Lindberg R. A., Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991 Jan;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R. C., Sohrabji F., Toran-Allerand C. D. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Scarisbrick I. A., Jones E. G., Isackson P. J. Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J Neurosci. 1993 Mar;13(3):875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecterson L. C., Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992 Sep;9(3):449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Sohrabji F., Miranda R. C., Toran-Allerand C. D. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994 Feb;14(2):459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Weinstein R., Rowe J. W., Maciag T., Fuhro R., Gardner R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3863–3866. doi: 10.1073/pnas.79.12.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L., Tsoulfas P., Martin-Zanca D., Gilbert D. J., Jenkins N. A., Copeland N. G., Parada L. F. trkC, a receptor for neurotrophin-3, is widely expressed in the developing nervous system and in non-neuronal tissues. Development. 1993 Jun;118(2):463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Tsoulfas P., Soppet D., Escandon E., Tessarollo L., Mendoza-Ramirez J. L., Rosenthal A., Nikolics K., Parada L. F. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993 May;10(5):975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- Tuttle J. B., Etheridge R., Creedon D. J. Receptor-mediated stimulation and inhibition of nerve growth factor secretion by vascular smooth muscle. Exp Cell Res. 1993 Oct;208(2):350–361. doi: 10.1006/excr.1993.1256. [DOI] [PubMed] [Google Scholar]
- Ueno H., Colbert H., Escobedo J. A., Williams L. T. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991 May 10;252(5007):844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- Valenzuela D. M., Maisonpierre P. C., Glass D. J., Rojas E., Nuñez L., Kong Y., Gies D. R., Stitt T. N., Ip N. Y., Yancopoulos G. D. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993 May;10(5):963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Wetmore C., Ernfors P., Persson H., Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990 Aug;109(2):141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Friedman P. L., Larhammar D., Persson H., Gonzalez-Carvajal M., Holets V. R. Rat beta-nerve growth factor sequence and site of synthesis in the adult hippocampus. J Neurosci Res. 1988 Aug;20(4):403–410. doi: 10.1002/jnr.490200402. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Vogel K. S., Davies A. M. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992 Jul;9(1):139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]