Abstract
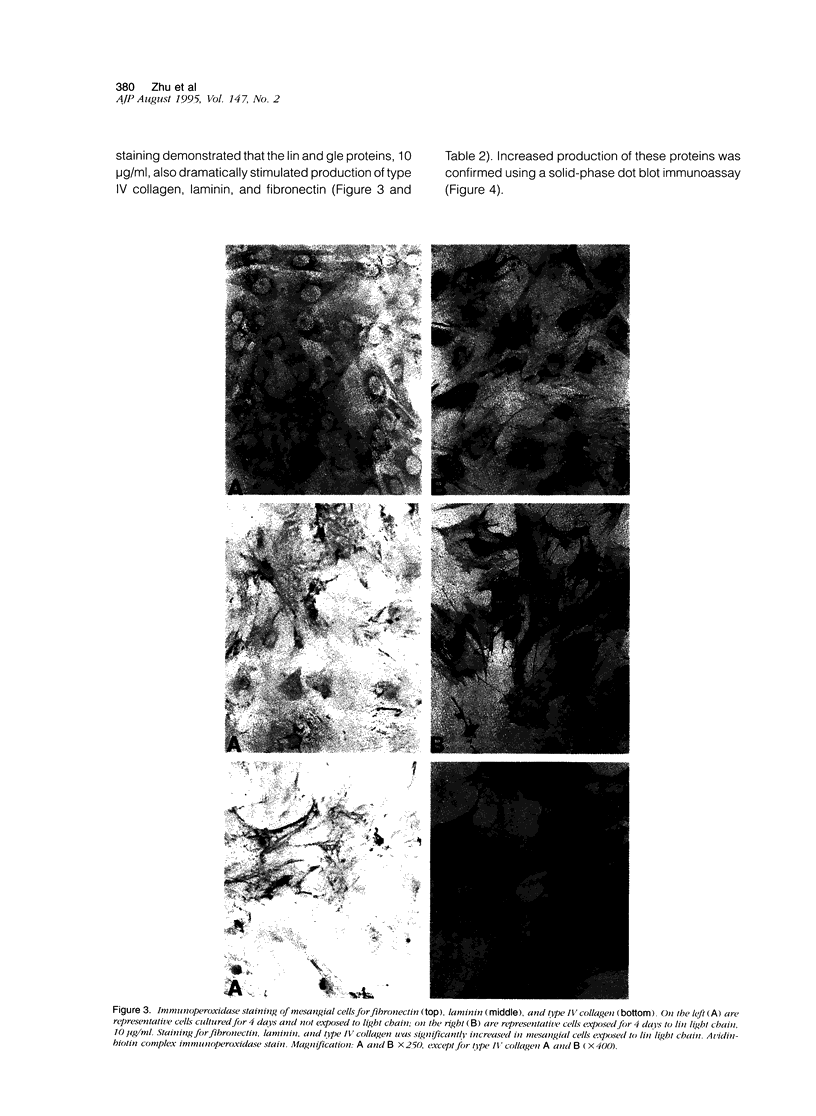
The glomerulopathy of monoclonal immunoglobulin light chain deposition disease is a progressive disorder characterized by accumulation of monoclonal light chains and matrix proteins in the mesangium. To define the role of light chains in this process, cultured rat mesangial cells were exposed to different light chains and human albumin. Two light chains were purified from the urine of patients who had biopsy-proven light chain deposition disease. These proteins inhibited mesangial cell proliferation and increased production of matrix proteins, including type IV collagen, laminin, and fibronectin. By immunocytochemistry and bioassay, transforming growth factor-beta (TGF-beta) production and activity increased when mesangial cells were exposed to these proteins. Furthermore, anti-TGF-beta antibody abolished the inhibition of cell proliferation and the increase of extracellular matrix protein production caused by these light chains. These findings were not observed in mesangial cells exposed to human albumin and two other light chains previously characterized to be tubulopathic. We concluded that the glomerulopathic light chains increased TGF-beta, which inhibited mesangial cell proliferation and increased matrix protein production. Together with overexpression of TGF-beta in affected glomeruli of light chain deposition disease, light chain-mediated stimulation of mesangial cells to produce TGF-beta appears to be a key pathological mechanism of this disease.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayo S. H., Radnik R. A., Garoni J. A., Glass W. F., 2nd, Kreisberg J. I. High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol. 1990 Jun;136(6):1339–1348. [PMC free article] [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A. Cytokines in kidney disease: the role of transforming growth factor-beta. Am J Kidney Dis. 1993 Jul;22(1):105–113. doi: 10.1016/s0272-6386(12)70175-0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M. E., Kim E. G., Huang Q., Ballermann B. J. Rat mesangial cell hypertrophy in response to transforming growth factor-beta 1. Kidney Int. 1993 Nov;44(5):948–958. doi: 10.1038/ki.1993.336. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng E., Floege J., Young B. A., Couser W. G., Johnson R. J. Does extracellular matrix expansion in glomerular disease require mesangial cell proliferation? Kidney Int Suppl. 1994 Feb;45:S45–S47. [PubMed] [Google Scholar]
- Grande J., Melder D., Zinsmeister A., Killen P. Transforming growth factor-beta 1 induces collagen IV gene expression in NIH-3T3 cells. Lab Invest. 1993 Oct;69(4):387–395. [PubMed] [Google Scholar]
- Harper P. A., Robinson J. M., Hoover R. L., Wright T. C., Karnovsky M. J. Improved methods for culturing rat glomerular cells. Kidney Int. 1984 Dec;26(6):875–880. doi: 10.1038/ki.1984.231. [DOI] [PubMed] [Google Scholar]
- Herrera G. A., Shultz J. J., Soong S. J., Sanders P. W. Growth factors in monoclonal light-chain--related renal diseases. Hum Pathol. 1994 Sep;25(9):883–892. doi: 10.1016/0046-8177(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Herrera G. A., Turbat-Herrera E. A., Viale G., dell'Orto P., Lott R. L., Colombi R., Alexander R. W., Coggi G. Ultrastructural immunolabeling in renal diseases. Past, present and future expectations. Pathol Immunopathol Res. 1987;6(1):51–63. doi: 10.1159/000157041. [DOI] [PubMed] [Google Scholar]
- Huang Z. Q., Kirk K. A., Connelly K. G., Sanders P. W. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J Clin Invest. 1993 Dec;92(6):2975–2983. doi: 10.1172/JCI116920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaname S., Uchida S., Ogata E., Kurokawa K. Autocrine secretion of transforming growth factor-beta in cultured rat mesangial cells. Kidney Int. 1992 Dec;42(6):1319–1327. doi: 10.1038/ki.1992.423. [DOI] [PubMed] [Google Scholar]
- Kyle R. A. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975 Jan;50(1):29–40. [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Stauffer J. W., Doi T., Agodoa L. Y., Striker G. E. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989 Apr;83(4):1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S., Kirk K. L., Bubien J. K., Oh Y., Hu P., Yue G., Shoemaker R., Cragoe E. J., Jr, Benos D. J. Immunocytochemical and functional characterization of Na+ conductance in adult alveolar pneumocytes. Am J Physiol. 1992 May;262(5 Pt 1):C1228–C1238. doi: 10.1152/ajpcell.1992.262.5.C1228. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirani C. L., Silva F., D'Agati V., Chander P., Striker L. M. Renal lesions in plasma cell dyscrasias: ultrastructural observations. Am J Kidney Dis. 1987 Sep;10(3):208–221. doi: 10.1016/s0272-6386(87)80176-2. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Chen A., Booker B. B., Galla J. H. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J Clin Invest. 1988 Dec;82(6):2086–2096. doi: 10.1172/JCI113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Galla J. H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987 Dec;32(6):851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Kirk K. A., Old C. W., Galla J. H. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab Invest. 1991 Apr;64(4):527–537. [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A. Monoclonal immunoglobulin light chain-related renal diseases. Semin Nephrol. 1993 May;13(3):324–341. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Schulze-Lohoff E., Weber M., Goodman S. L. Interactions between glomerular mesangial cells, cytokines, and extracellular matrix. J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S126–S131. doi: 10.1681/ASN.V210s126. [DOI] [PubMed] [Google Scholar]
- Wolf G., Sharma K., Chen Y., Ericksen M., Ziyadeh F. N. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-beta. Kidney Int. 1992 Sep;42(3):647–656. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura T., Noble N. A., Ruoslahti E., Border W. A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh F. N., Sharma K., Ericksen M., Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994 Feb;93(2):536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]






