Abstract
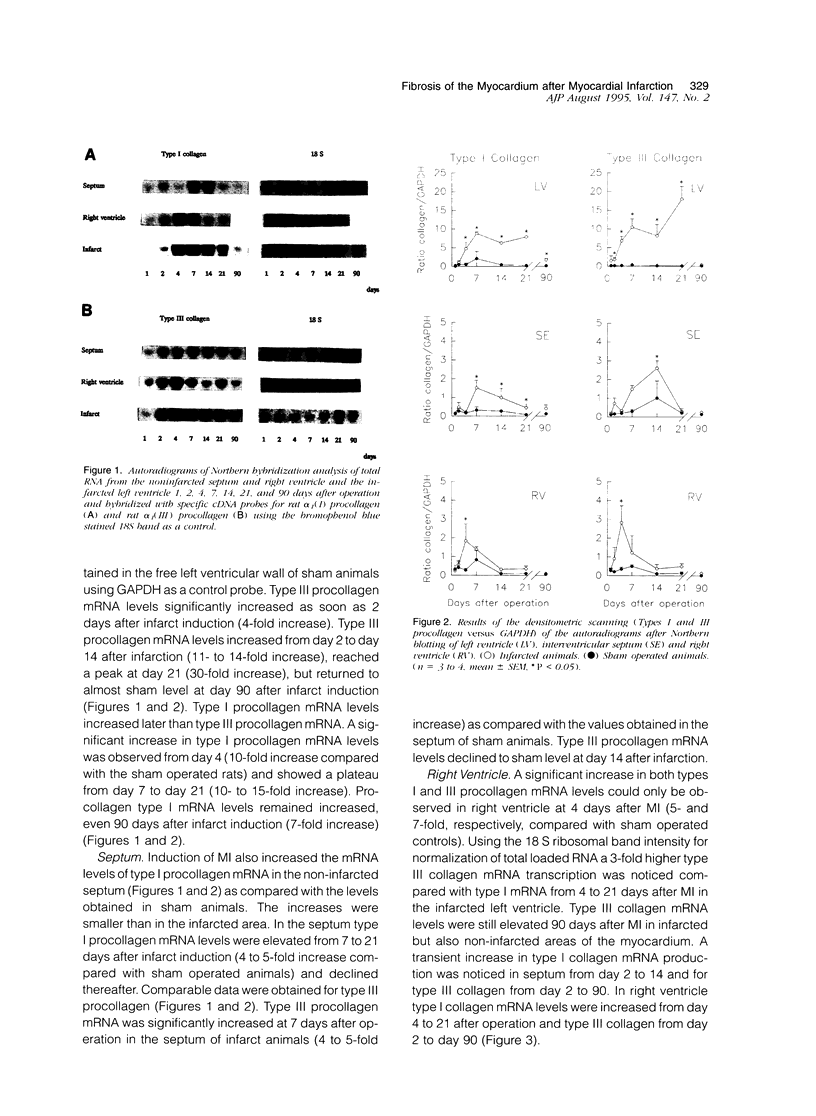
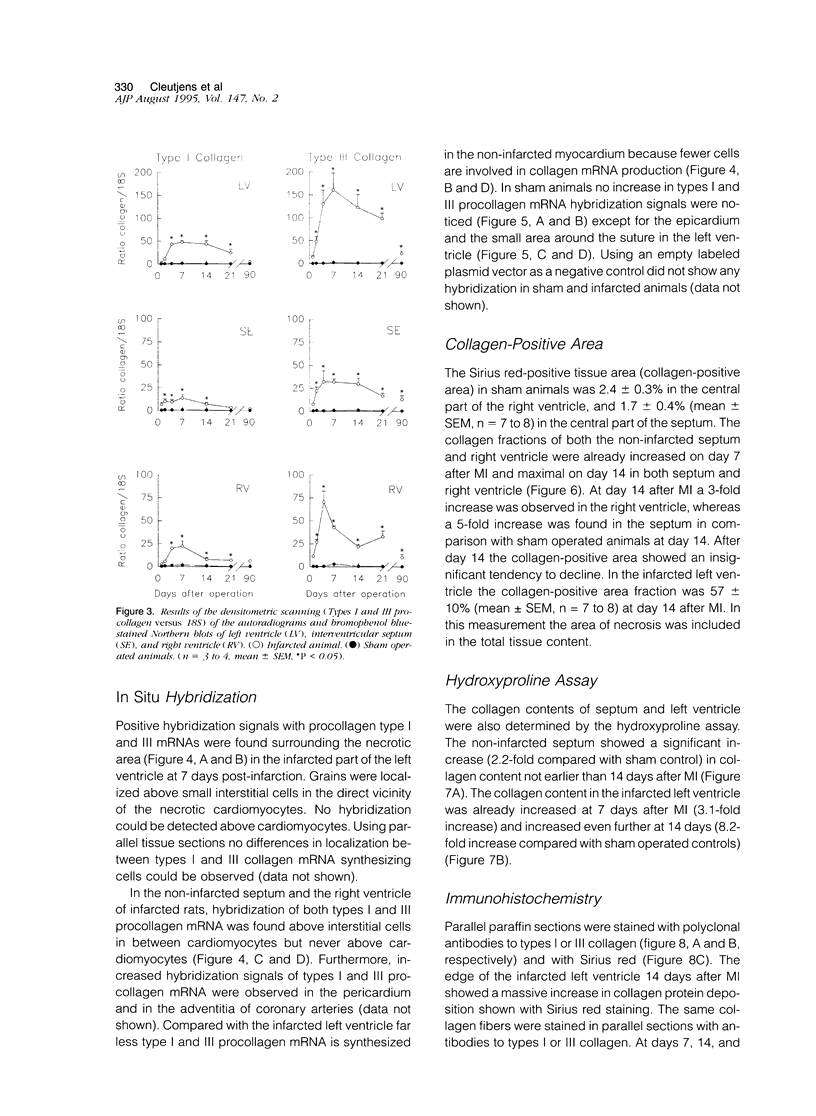
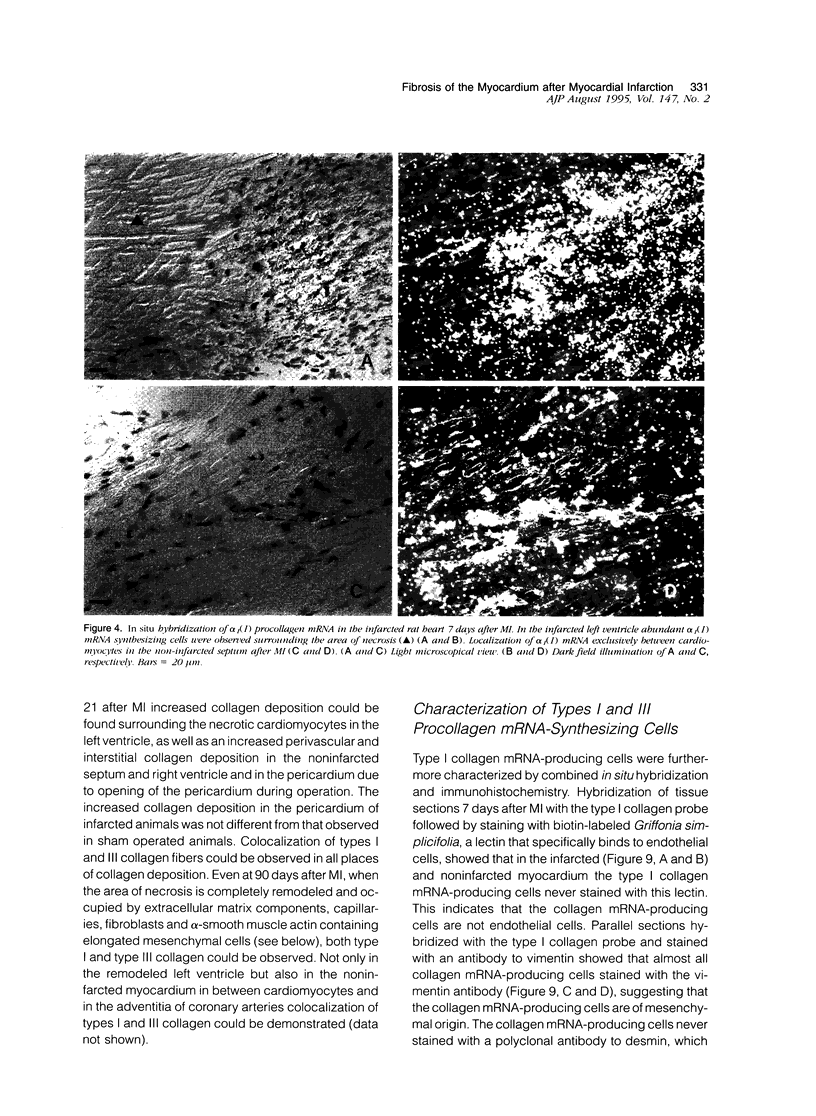
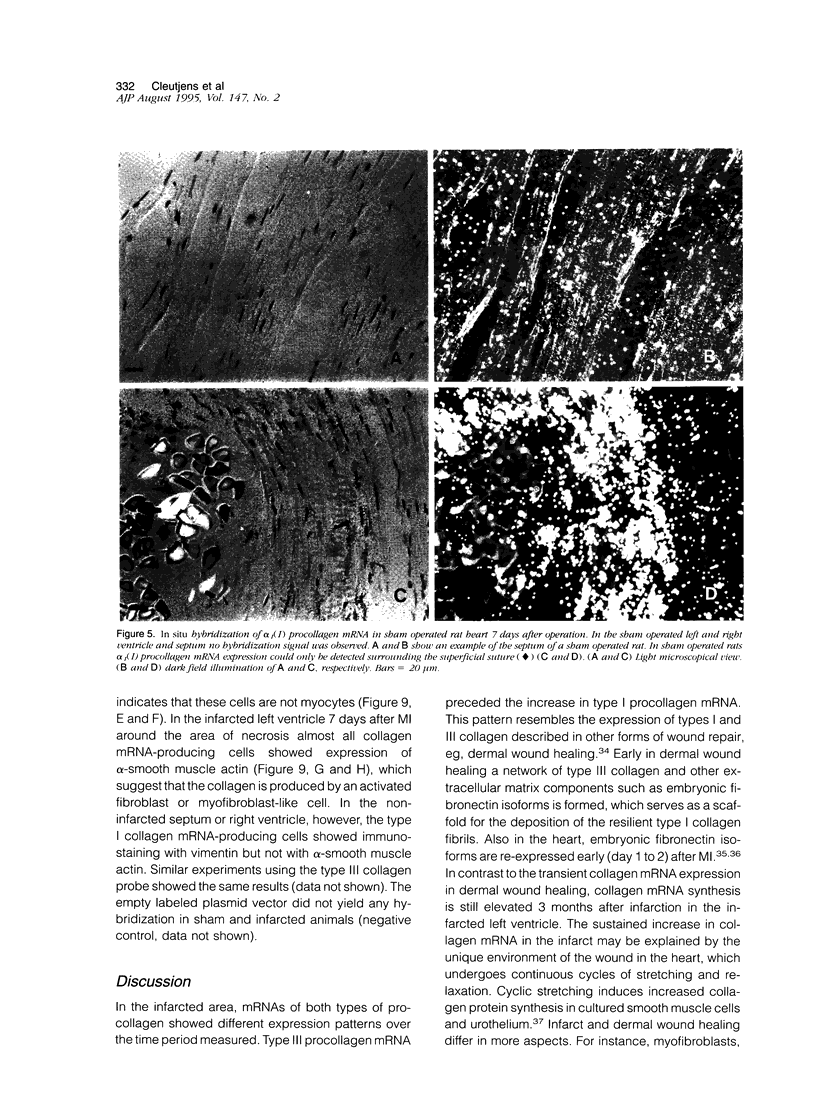
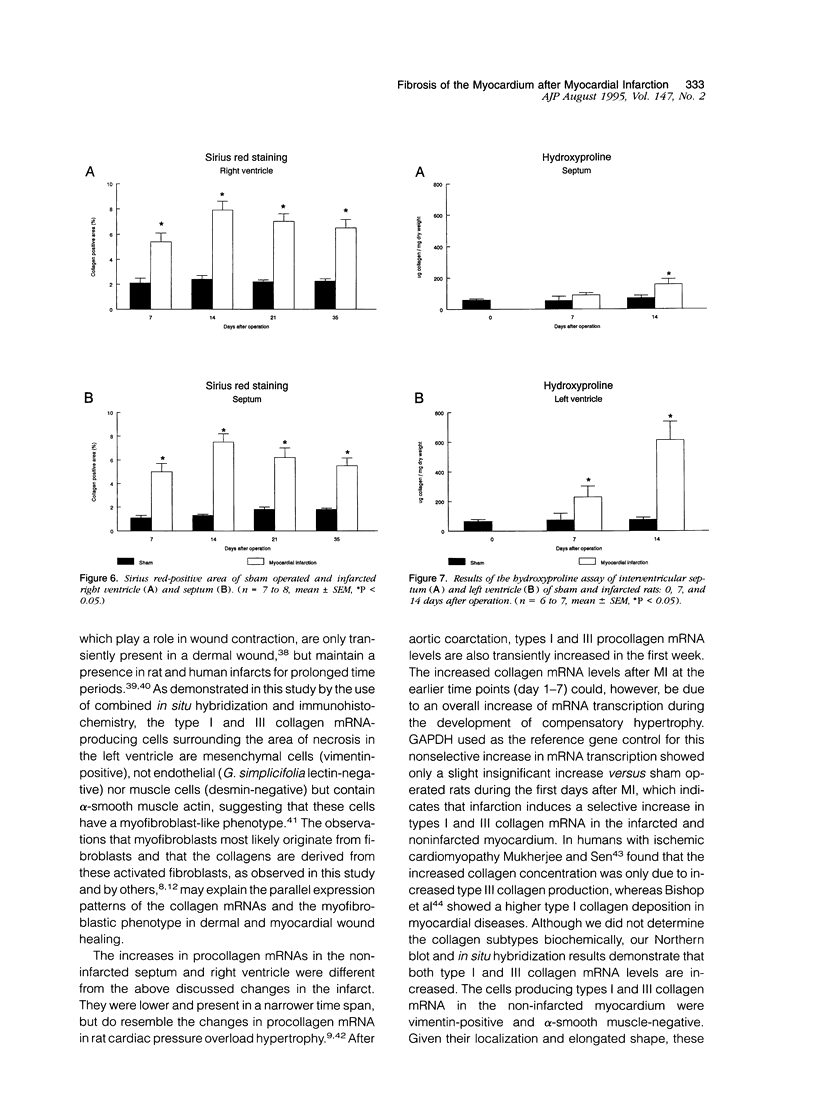
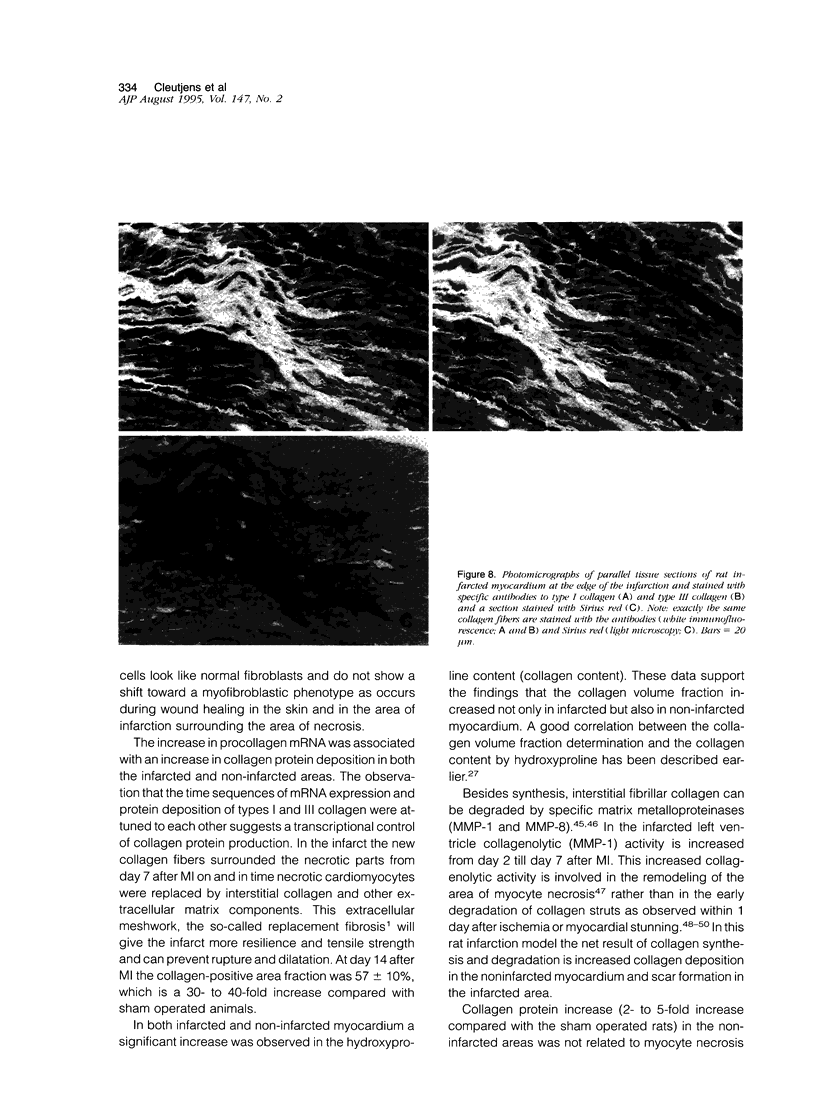
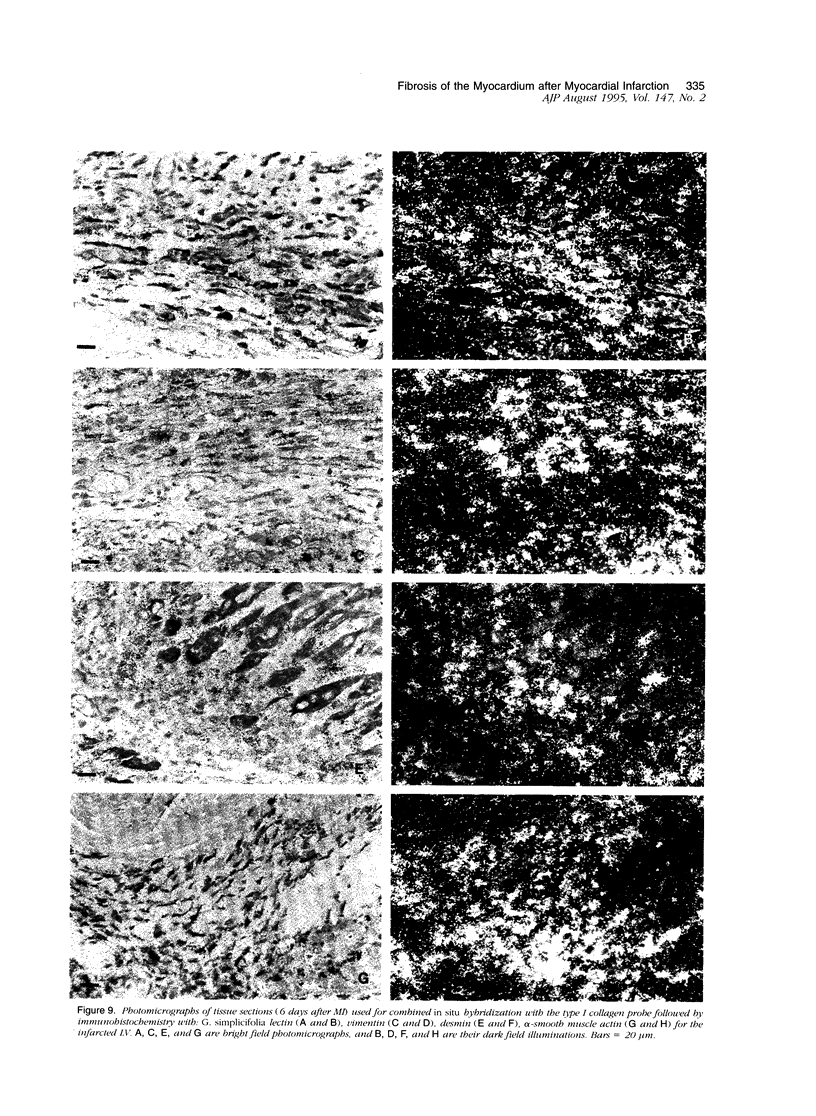
In this study changes in the amount and distribution of types I and III collagen mRNA and protein were investigated in the rat heart after induction of a left ventricular myocardial infarction (MI). Sham operated rats served as controls. The animals were sacrificed at different time intervals after operation. Northern blotting of cardiac RNA and hybridization with cDNA probes for types I and III procollagen revealed a 5- to 15-fold increase in the infarcted left ventricle. Type III procollagen mRNA levels were already increased at day 2 after MI, whereas type I procollagen mRNA followed this response at day 4 after MI. This increase was sustained for at least 21 days in the infarcted left ventricle for type III procollagen mRNA, whereas type 1 procollagen mRNA levels were still elevated at 90 days after MI. In the noninfarcted right ventricle a 5- to 7-fold increase was observed for both type I and type III procollagen mRNA levels, but only at day 4 after MI. In the non-infarcted septum a transient increase was observed for type I procollagen mRNA from day 7-21 (4- to 5-fold increase) and a decline to sham levels thereafter. In the septum type III procollagen mRNA levels were only elevated at 7 days after MI (4- to 5-fold increase) compared with sham operated controls. In situ hybridization with the same types I and III procollagen probes showed procollagen mRNA-producing cells in the infarcted area around necrotic cardiomyocytes, and in the interstitial cells in the non-infarcted part of the myocardium. No labeling was detected above cardiomyocytes. Combined in situ hybridization and immunohistochemistry showed that the collagen mRNA producing cells have a myofibroblast-like phenotype in the infarcted myocardium and are fibroblasts in the noninfarcted septum and right ventricle. The increase in types I and III procollagen mRNA in both infarcted and non-infarcted myocardium was followed by an increased collagen deposition, measured by computerized morphometry on sirius red-stained tissue sections as well as by the hydroxyproline assay. In the non-infarcted septum and right ventricle the collagen-positive area was maximal at day 14 (3- to 5-fold increase compared with sham operated controls) and slightly declined at day 21. In the infarcted myocardium the collagen-positive area was 57 +/- 10% at day 14 after MI. Hydroxyproline contents were significantly increased in the noninfarcted septum.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskin L., Howard P. S., Macarak E. Effect of physical forces on bladder smooth muscle and urothelium. J Urol. 1993 Aug;150(2 Pt 2):601–607. doi: 10.1016/s0022-5347(17)35560-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. E., Greenbaum R., Gibson D. G., Yacoub M., Laurent G. J. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990 Oct;22(10):1157–1165. doi: 10.1016/0022-2828(90)90079-h. [DOI] [PubMed] [Google Scholar]
- Brilla C. G., Pick R., Tan L. B., Janicki J. S., Weber K. T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990 Dec;67(6):1355–1364. doi: 10.1161/01.res.67.6.1355. [DOI] [PubMed] [Google Scholar]
- Cannon R. O., 3rd, Butany J. W., McManus B. M., Speir E., Kravitz A. B., Bolli R., Ferrans V. J. Early degradation of collagen after acute myocardial infarction in the rat. Am J Cardiol. 1983 Aug;52(3):390–395. doi: 10.1016/0002-9149(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Casscells W., Kimura H., Sanchez J. A., Yu Z. X., Ferrans V. J. Immunohistochemical study of fibronectin in experimental myocardial infarction. Am J Pathol. 1990 Oct;137(4):801–810. [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. B., Borg T. K. The collagen network of the heart. Lab Invest. 1979 Mar;40(3):364–372. [PubMed] [Google Scholar]
- Chiariello M., Ambrosio G., Cappelli-Bigazzi M., Perrone-Filardi P., Brigante F., Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol. 1986 Mar;18(3):283–290. doi: 10.1016/s0022-2828(86)80410-2. [DOI] [PubMed] [Google Scholar]
- Cleutjens J. P., Havenith M. G., Beek C., Vallinga M., Ten Kate J., Bosman F. T. Origin of basement membrane type IV collagen in xenografted human epithelial tumor cell lines. Am J Pathol. 1990 May;136(5):1165–1172. [PMC free article] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering C. W., Jalil J. E., Janicki J. S., Pick R., Aghili S., Abrahams C., Weber K. T. Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res. 1988 Oct;22(10):686–695. doi: 10.1093/cvr/22.10.686. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Blumenfeld O. O., Seifter S., Buttrick P. M., Leinwand L. A., Robinson T. F., Zern M. A., Giambrone M. A. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989 Jan;21(1):103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Tomek R., Sukhatme V. P., Woods C., Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res. 1991 Aug;69(2):483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- Eleftheriades E. G., Durand J. B., Ferguson A. G., Engelmann G. L., Jones S. B., Samarel A. M. Regulation of procollagen metabolism in the pressure-overloaded rat heart. J Clin Invest. 1993 Mar;91(3):1113–1122. doi: 10.1172/JCI116270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriades E. G., Ferguson A. G., Samarel A. M. Cyclosporine A has no direct effect on collagen metabolism by cardiac fibroblasts in vitro. Circulation. 1993 Apr;87(4):1368–1377. doi: 10.1161/01.cir.87.4.1368. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978 Jan;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Gressner A. M. Hepatic fibrogenesis: the puzzle of interacting cells, fibrogenic cytokines, regulatory loops, and extracellular matrix molecules. Z Gastroenterol. 1992 Mar;30 (Suppl 1):5–16. [PubMed] [Google Scholar]
- James J., Bosch K. S., Zuyderhoudt F. M., Houtkooper J. M., van Gool J. Histophotometric estimation of volume density of collagen as an indication of fibrosis in rat liver. Histochemistry. 1986;85(2):129–133. doi: 10.1007/BF00491759. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Vaheri A., Roberts P. J., Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest. 1980 Jul;43(1):47–51. [PubMed] [Google Scholar]
- Laurent G. J. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987 Jan;252(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- Lenkiewicz J. E., Davies M. J., Rosen D. Collagen in human myocardium as a function of age. Cardiovasc Res. 1972 Sep;6(5):549–555. doi: 10.1093/cvr/6.5.549. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K., Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991 Apr;68(4):905–921. doi: 10.1161/01.res.68.4.905. [DOI] [PubMed] [Google Scholar]
- Litwin S. E., Litwin C. M., Raya T. E., Warner A. L., Goldman S. Contractility and stiffness of noninfarcted myocardium after coronary ligation in rats. Effects of chronic angiotensin converting enzyme inhibition. Circulation. 1991 Mar;83(3):1028–1037. doi: 10.1161/01.cir.83.3.1028. [DOI] [PubMed] [Google Scholar]
- Merx W., Yoon M. S., Han J. The role of local disparity in conduction and recovery time on ventricular vulnerability to fibrillation. Am Heart J. 1977 Nov;94(5):603–610. doi: 10.1016/s0002-8703(77)80130-0. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Toman D., De Crombrugghe B., Vuorio E. Specific hybridization probes for mouse type I, II, III and IX collagen mRNAs. Biochim Biophys Acta. 1991 Jun 13;1089(2):241–243. doi: 10.1016/0167-4781(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Michel J. B., Lattion A. L., Salzmann J. L., Cerol M. L., Philippe M., Camilleri J. P., Corvol P. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ Res. 1988 Apr;62(4):641–650. doi: 10.1161/01.res.62.4.641. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest. 1991 Oct;88(4):1141–1146. doi: 10.1172/JCI115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering J. G., Boughner D. R. Quantitative assessment of the age of fibrotic lesions using polarized light microscopy and digital image analysis. Am J Pathol. 1991 May;138(5):1225–1231. [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L., Petersen O. W. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993 Jun;68(6):696–707. [PubMed] [Google Scholar]
- Sato S., Ashraf M., Millard R. W., Fujiwara H., Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol. 1983 Apr;15(4):261–275. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Schoemaker R. G., Urquhart J., Debets J. J., Struyker Boudier H. A., Smits J. F. Acute hemodynamic effects of coronary artery ligation in conscious rats. Basic Res Cardiol. 1990 Jan-Feb;85(1):9–20. doi: 10.1007/BF01907010. [DOI] [PubMed] [Google Scholar]
- Sekita S., Katagiri T., Sasai Y., Takeda K. Studies on collagen in the experimental myocardial infarction. Jpn Circ J. 1985 Feb;49(2):171–178. doi: 10.1253/jcj.49.171. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Guriev S. B., Irgashev S. B., Koteliansky V. E. Immunofluorescent identification of fibronectin and fibrinogen/fibrin in experimental myocardial infarction. J Mol Cell Cardiol. 1990 May;22(5):533–541. doi: 10.1016/0022-2828(90)90955-2. [DOI] [PubMed] [Google Scholar]
- Smits J. F., Cleutjens J. P., van Krimpen C., Schoemaker R. G., Daemen M. J. Cardiac remodeling in hypertension and following myocardial infarction: effects of arteriolar vasodilators. Basic Res Cardiol. 1991;86 (Suppl 1):133–139. [PubMed] [Google Scholar]
- Smits J. F., van Krimpen C., Schoemaker R. G., Cleutjens J. P., Daemen M. J. Angiotensin II receptor blockade after myocardial infarction in rats: effects on hemodynamics, myocardial DNA synthesis, and interstitial collagen content. J Cardiovasc Pharmacol. 1992;20(5):772–778. [PubMed] [Google Scholar]
- Sun Y., Cleutjens J. P., Diaz-Arias A. A., Weber K. T. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994 Sep;28(9):1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Barry A. C., Factor S. M. Collagen degradation in ischaemic rat hearts. Biochem J. 1990 Jan 1;265(1):233–241. doi: 10.1042/bj2650233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Volders P. G., Willems I. E., Cleutjens J. P., Arends J. W., Havenith M. G., Daemen M. J. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol. 1993 Nov;25(11):1317–1323. doi: 10.1006/jmcc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Vracko R., Thorning D., Frederickson R. G. Connective tissue cells in healing rat myocardium. A study of cell reactions in rhythmically contracting environment. Am J Pathol. 1989 May;134(5):993–1006. [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Weber K. T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989 Jun;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Pick R., Jalil J. E., Janicki J. S., Carroll E. P. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989 Dec;21 (Suppl 5):121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- Whittaker P., Boughner D. R., Kloner R. A. Role of collagen in acute myocardial infarct expansion. Circulation. 1991 Nov;84(5):2123–2134. doi: 10.1161/01.cir.84.5.2123. [DOI] [PubMed] [Google Scholar]
- Willems I. E., Havenith M. G., De Mey J. G., Daemen M. J. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994 Oct;145(4):868–875. [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yao J., Eghbali M. Decreased collagen gene expression and absence of fibrosis in thyroid hormone-induced myocardial hypertrophy. Response of cardiac fibroblasts to thyroid hormone in vitro. Circ Res. 1992 Oct;71(4):831–839. doi: 10.1161/01.res.71.4.831. [DOI] [PubMed] [Google Scholar]
- Zhao M. J., Zhang H., Robinson T. F., Factor S. M., Sonnenblick E. H., Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional ("stunned") but viable myocardium. J Am Coll Cardiol. 1987 Dec;10(6):1322–1334. doi: 10.1016/s0735-1097(87)80137-7. [DOI] [PubMed] [Google Scholar]
- van Krimpen C., Schoemaker R. G., Cleutjens J. P., Smits J. F., Struyker-Boudier H. A., Bosman F. T., Daemen M. J. Angiotensin I converting enzyme inhibitors and cardiac remodeling. Basic Res Cardiol. 1991;86 (Suppl 1):149–155. [PubMed] [Google Scholar]
- van Krimpen C., Smits J. F., Cleutjens J. P., Debets J. J., Schoemaker R. G., Struyker Boudier H. A., Bosman F. T., Daemen M. J. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat: effects of captopril. J Mol Cell Cardiol. 1991 Nov;23(11):1245–1253. doi: 10.1016/0022-2828(91)90082-w. [DOI] [PubMed] [Google Scholar]








