Abstract
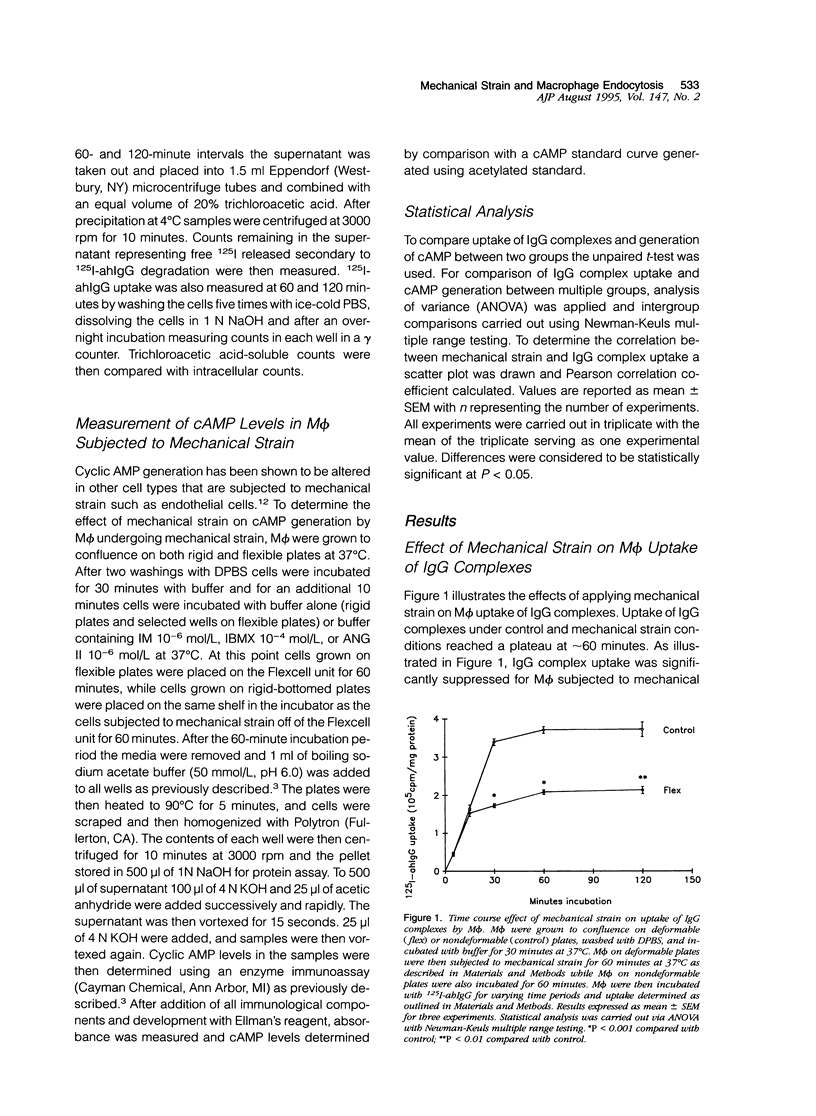
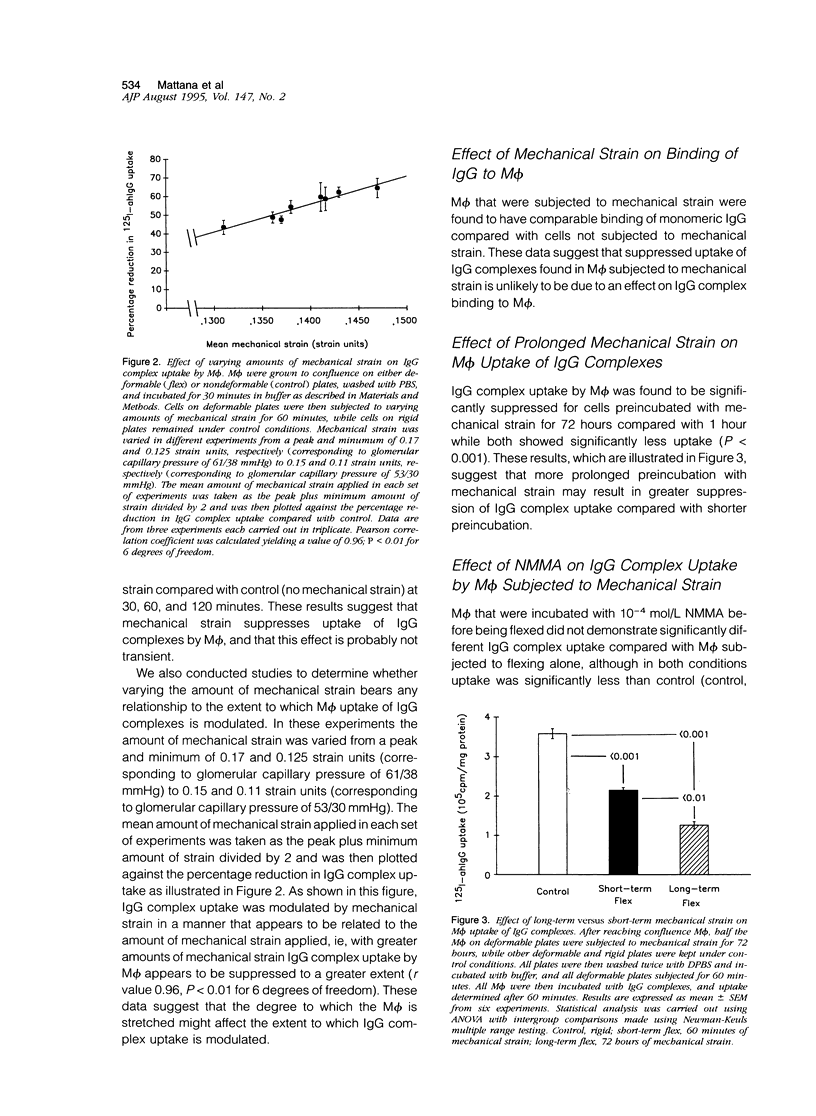
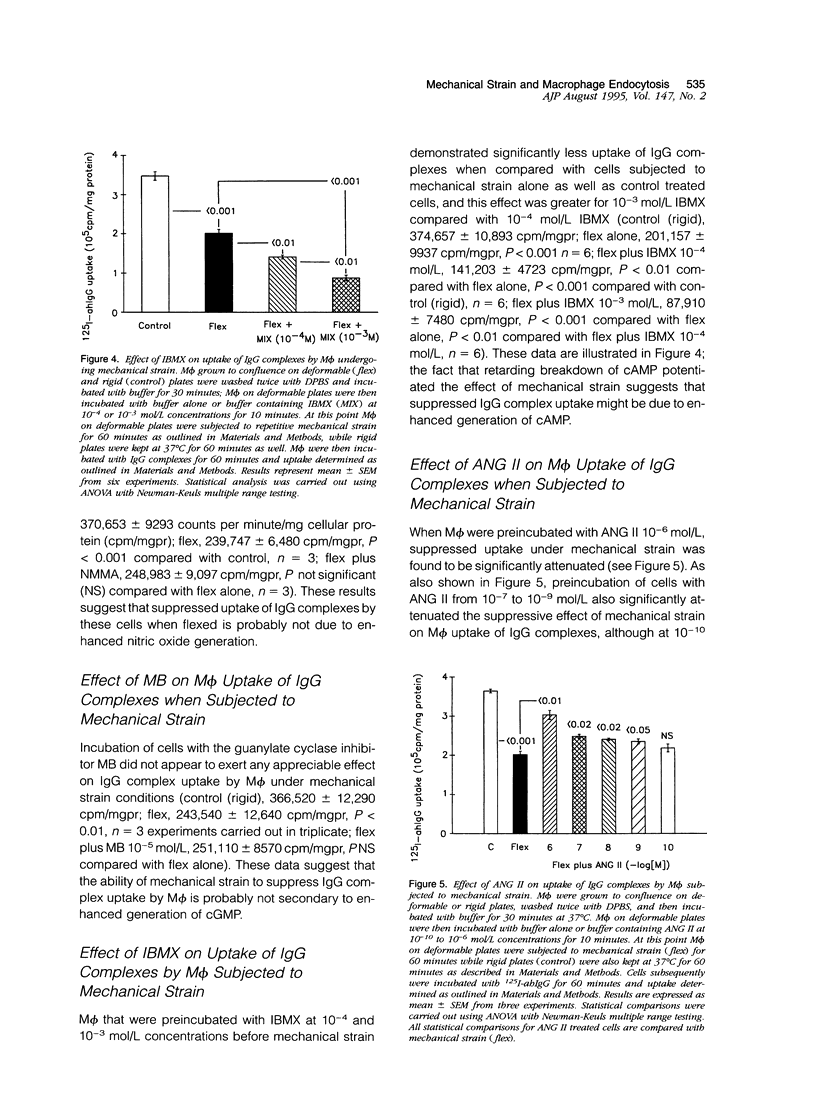
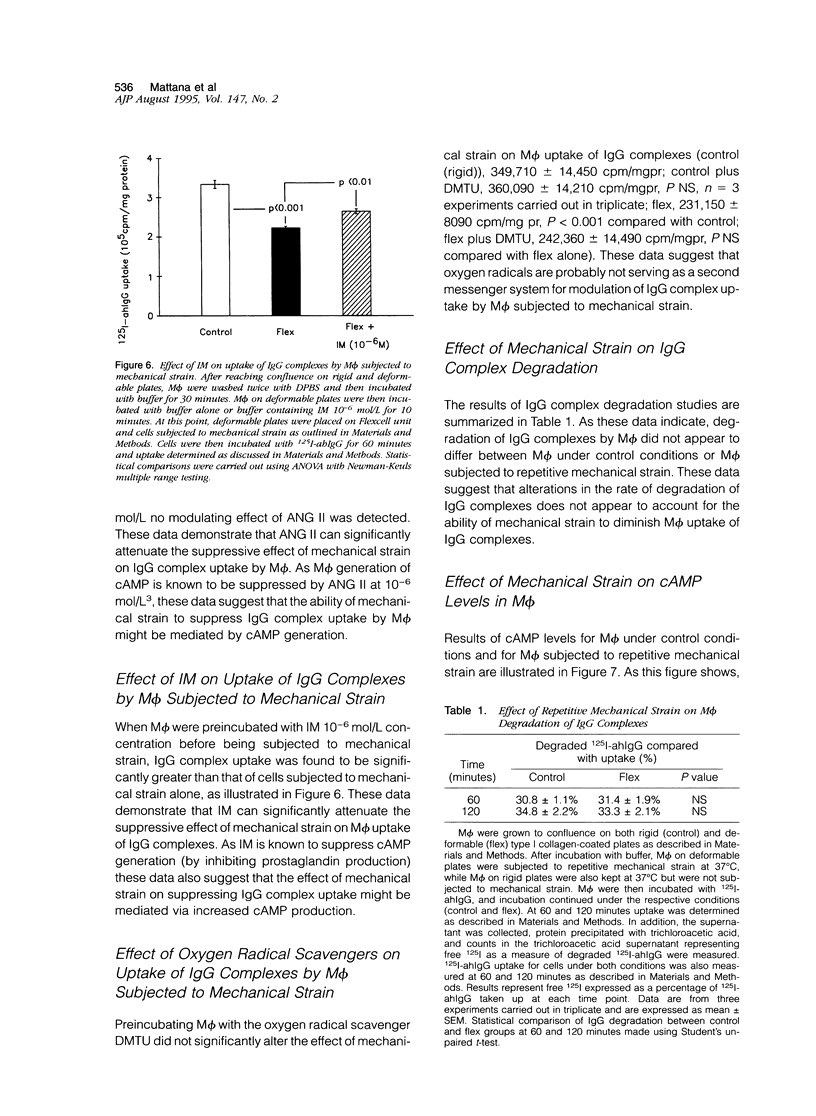
Uptake of immunoglobulin G (IgG) complexes by macrophages (M phi) may play an important role in disease states characterized by increased levels of circulating immune complexes. In sites such as the glomerular mesangium M phi may be subjected to repetitive mechanical strain, although in vitro studies of M phi endocytosis are typically carried out with cells grown on rigid surfaces. We undertook the present study to determine whether repetitive mechanical strain could modulate M phi endocytosis of IgG complexes. IgG complex uptake was significantly diminished in M phi that were subjected to repetitive mechanical strain using parameters corresponding to peak and minimal intraglomerular pressures compared with control, and uptake varied according to the amount of mechanical strain applied. There was no significant difference in surface binding of IgG between M phi subjected to strain and those not. Mechanical strain did not significantly influence the rate of IgG complex degradation. Inhibition of nitric oxide synthase and guanylate cyclase activity did not alter the effect of mechanical strain, although this effect was potentiated by 3-isobutyl-1-methylxanthine (IBMX). Angiotensin II, which has been shown to reduce adenosine 3',5'-cyclic monophosphate (cAMP) production in M phi, significantly attenuated the suppressive effect of mechanical strain on IgG complex uptake as well as another inhibitor of cAMP generation, indomethacin. Enzyme immunoassay demonstrated significantly enhanced levels of cAMP in M phi that were subjected to mechanical strain compared with control, an effect that was potentiated by IBMX and attenuated by angiotensin II and indomethacin. These results demonstrate that repetitive mechanical strain significantly reduces IgG complex uptake by M phi, most likely by enhancing cAMP synthesis. Such an effect might play a significant role in macromolecule handling by M phi in sites in which they are subjected to repetitive mechanical deformation such as the glomerular mesangium.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Rennke H. G., Brenner B. M. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986 Jun;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971 Aug;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Ding G., Frye J., Diamond I. P. Glomerular macrophages and the mesangial proliferative response in the experimental nephrotic syndrome. Am J Pathol. 1992 Oct;141(4):887–894. [PMC free article] [PubMed] [Google Scholar]
- Floege J., Alpers C. E., Burns M. W., Pritzl P., Gordon K., Couser W. G., Johnson R. J. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Lab Invest. 1992 Apr;66(4):485–497. [PubMed] [Google Scholar]
- Fogo A., Hawkins E. P., Berry P. L., Glick A. D., Chiang M. L., MacDonell R. C., Jr, Ichikawa I. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990 Jul;38(1):115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- Furness P. N. Immune complex degradation by cultured rat mesangial cells. J Pathol. 1993 Jun;170(2):197–203. doi: 10.1002/path.1711700216. [DOI] [PubMed] [Google Scholar]
- Green S. A., Plutner H., Mellman I. Biosynthesis and intracellular transport of the mouse macrophage Fc receptor. J Biol Chem. 1985 Aug 15;260(17):9867–9874. [PubMed] [Google Scholar]
- Harris R. C., Haralson M. A., Badr K. F. Continuous stretch-relaxation in culture alters rat mesangial cell morphology, growth characteristics, and metabolic activity. Lab Invest. 1992 May;66(5):548–554. [PubMed] [Google Scholar]
- Hostetter T. H., Olson J. L., Rennke H. G., Venkatachalam M. A., Brenner B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981 Jul;241(1):F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Nusbacher J., Anderson C. L. Fc receptor-mediated binding and endocytosis by human mononuclear phagocytes: monomeric IgG is not endocytosed by U937 cells and monocytes. J Cell Biol. 1985 Feb;100(2):558–564. doi: 10.1083/jcb.100.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latta H., Fligiel S. Mesangial fenestrations, sieving, filtration, and flow. Lab Invest. 1985 Jun;52(6):591–598. [PubMed] [Google Scholar]
- Letsou G. V., Rosales O., Maitz S., Vogt A., Sumpio B. E. Stimulation of adenylate cyclase activity in cultured endothelial cells subjected to cyclic stretch. J Cardiovasc Surg (Torino) 1990 Sep-Oct;31(5):634–639. [PubMed] [Google Scholar]
- Madri J. A., Marx M. Matrix composition, organization and soluble factors: modulators of microvascular cell differentiation in vitro. Kidney Int. 1992 Mar;41(3):560–565. doi: 10.1038/ki.1992.82. [DOI] [PubMed] [Google Scholar]
- Mattana J., Singhal P. C. Effects of atrial natriuretic peptide and cGMP on uptake of IgG complexes by macrophages. Am J Physiol. 1993 Jul;265(1 Pt 1):C92–C98. doi: 10.1152/ajpcell.1993.265.1.C92. [DOI] [PubMed] [Google Scholar]
- Mattana J., Singhal P. C. Effects of prostaglandin E2 and cyclic AMP on uptake of immunoglobulin G complexes by cultured macrophages. Eicosanoids. 1992;5(2):67–72. [PubMed] [Google Scholar]
- Mattana J., Singhal P. C. Macrophage Fc receptor activity modulates mesangial cell proliferation and matrix synthesis. Am J Physiol. 1994 Apr;266(4 Pt 2):F568–F575. doi: 10.1152/ajprenal.1994.266.4.F568. [DOI] [PubMed] [Google Scholar]
- Mattana J., Singhal P. C. Macrophage supernatants have both stimulatory and suppressive effects on mesangial cell proliferation. J Cell Physiol. 1993 Feb;154(2):289–293. doi: 10.1002/jcp.1041540211. [DOI] [PubMed] [Google Scholar]
- Monga G., Mazzucco G., di Belgiojoso G. B., Busnach G. The presence and possible role of monocyte infiltration in human chronic proliferative glomerulonephritides. Light microscopic, immunofluorescence, and histochemical correlations. Am J Pathol. 1979 Feb;94(2):271–284. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirth R., Singhal P., Diamond B., Hays R. M., Lobmeyer L., Clay K., Schlondorff D. Evidence for immunoglobulin Fc receptor-mediated prostaglandin2 and platelet-activating factor formation by cultured rat mesangial cells. J Clin Invest. 1988 Sep;82(3):936–944. doi: 10.1172/JCI113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesek-Diamond I., Ding G., Frye J., Diamond J. R. Macrophages mediate adverse effects of cholesterol feeding in experimental nephrosis. Am J Physiol. 1992 Nov;263(5 Pt 2):F776–F783. doi: 10.1152/ajprenal.1992.263.5.F776. [DOI] [PubMed] [Google Scholar]
- Saito T., Atkins R. C. Contribution of mononuclear leucocytes to the progression of experimental focal glomerular sclerosis. Kidney Int. 1990 Apr;37(4):1076–1083. doi: 10.1038/ki.1990.88. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Shuldiner M., Hora K., Xing Y., Shan Z., Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993 Sep;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriano J., Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3':5'-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest. 1994 Oct;94(4):1629–1636. doi: 10.1172/JCI117505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F. The mesangial phagocyte and its regulation of contractile cell biology. J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S74–S82. doi: 10.1681/ASN.V210s74. [DOI] [PubMed] [Google Scholar]
- Singhal P. C., Ding G. H., DeCandido S., Franki N., Hays R. M., Schlondorff D. Endocytosis by cultured mesangial cells and associated changes in prostaglandin E2 synthesis. Am J Physiol. 1987 Apr;252(4 Pt 2):F627–F634. doi: 10.1152/ajprenal.1987.252.4.F627. [DOI] [PubMed] [Google Scholar]
- Singhal P. C., Gupta S., Shen Z., Schlondorff D. Effects of PGE2 and a thromboxane A2 analogue on uptake of IgG complexes and LDL by mesangial cells. Am J Physiol. 1991 Sep;261(3 Pt 2):F537–F544. doi: 10.1152/ajprenal.1991.261.3.F537. [DOI] [PubMed] [Google Scholar]
- Singhal P. C., Hays R. M. Actin filament morphology in living and nonliving cultured mesangial cells: formation and dissolution. Nephron. 1988;50(1):28–33. doi: 10.1159/000185112. [DOI] [PubMed] [Google Scholar]
- Singhal P. C., Pan C., Gibbons N. Effect of morphine on uptake of immunoglobulin G complexes by mesangial cells and macrophages. Am J Physiol. 1993 May;264(5 Pt 2):F859–F866. doi: 10.1152/ajprenal.1993.264.5.F859. [DOI] [PubMed] [Google Scholar]
- Singhal P. C., Santiago A., Satriano J., Hays R. M., Schlondorff D. Effects of vasoactive agents on uptake of immunoglobulin G complexes by mesangial cells. Am J Physiol. 1990 Mar;258(3 Pt 2):F589–F596. doi: 10.1152/ajprenal.1990.258.3.F589. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goor H., van der Horst M. L., Fidler V., Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992 May;66(5):564–571. [PubMed] [Google Scholar]


