Abstract
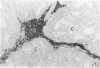
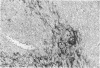
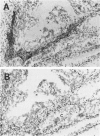
The expression of P450 aromatase and other steroidogenic enzymes were evaluated in 42 endometrioid endometrial carcinomas, 23 endometrial hyperplasias, and 7 normal endometrial specimens. These findings were correlated with clinicopathological findings to elucidate the possible biological significance of in situ estrogen production in the development of human endometrial carcinoma. Only weak aromatase immunoreactivity was observed in vascular walls and myometrial cells. In contrast, strong aromatase stromal immunoreactivity was observed in 28 of 42 (66.7%) endometrial carcinomas. However, no stromal immunoreactivity was seen in normal or hyperplastic endometrial specimens. Immunoreactivity in the carcinoma stromal cells was significantly increased at sites of invasion. These aromatase-positive cells were immunohistochemically negative for other steroidogenic enzymes involved in estrogen biosynthesis. In situ hybridization studies revealed aromatase mRNA hybridization signals in stromal cells but not in carcinoma cells. The distribution of aromatase mRNA correlated well with the immunohistochemical localization of aromatase enzyme. Quantitation of aromatase activity demonstrated 8.75 +/- 2.75 pmol/hour/mg of protein for endometrial carcinomas (22 specimens) and 0.98 +/- 1.95 pmol/hour/mg of protein for normal endometrial specimens (4 specimens). Aromatase activity was found in both estrogen receptor-positive and -negative endometrial carcinomas. Aromatase did not vary with respect to the menopausal status of patients with endometrial carcinoma. These results suggest that estrogen is produced in situ in endometrial carcinoma but not in benign endometrial lesions. Such locally synthesized estrogen may act on carcinoma cells in a paracrine fashion to promote tumor growth. Additional investigations are necessary, but increased aromatase expression in the stromal cells of endometrial carcinoma may therefore play an important role in the development of human endometrioid endometrial carcinoma.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxendale P. M., Reed M. J., James V. H. Inability of human endometrium or myometrium to aromatize androstenedione. J Steroid Biochem. 1981 Mar;14(3):305–306. doi: 10.1016/0022-4731(81)90140-0. [DOI] [PubMed] [Google Scholar]
- Bulun S. E., Mahendroo M. S., Simpson E. R. Polymerase chain reaction amplification fails to detect aromatase cytochrome P450 transcripts in normal human endometrium or decidua. J Clin Endocrinol Metab. 1993 Jun;76(6):1458–1463. doi: 10.1210/jcem.76.6.7684741. [DOI] [PubMed] [Google Scholar]
- Bulun S. E., Rosenthal I. M., Brodie A. M., Inkster S. E., Zeller W. P., DiGeorge A. M., Frasier S. D., Kilgore M. W., Simpson E. R. Use of tissue-specific promoters in the regulation of aromatase cytochrome P450 gene expression in human testicular and ovarian sex cord tumors, as well as in normal fetal and adult gonads. J Clin Endocrinol Metab. 1993 Dec;77(6):1616–1621. doi: 10.1210/jcem.77.6.8263150. [DOI] [PubMed] [Google Scholar]
- Harada N. Novel properties of human placental aromatase as cytochrome P-450: purification and characterization of a unique form of aromatase. J Biochem. 1988 Jan;103(1):106–113. doi: 10.1093/oxfordjournals.jbchem.a122213. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Ninomiya Y., Hayashi K., Linsenmayer T. F., Olsen B. R., Trelstad R. L. Secretion of collagen types I and II by epithelial and endothelial cells in the developing chick cornea demonstrated by in situ hybridization and immunohistochemistry. Development. 1988 May;103(1):27–36. doi: 10.1242/dev.103.1.27. [DOI] [PubMed] [Google Scholar]
- Judd H. L., Davidson B. J., Frumar A. M., Shamonki I. M., Lagasse L. D., Ballon S. C. Serum androgens and estrogens in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1980 Apr 1;136(7):859–871. doi: 10.1016/0002-9378(80)91043-1. [DOI] [PubMed] [Google Scholar]
- Kelsey J. L., LiVolsi V. A., Holford T. R., Fischer D. B., Mostow E. D., Schwartz P. E., O'Connor T., White C. A case-control study of cancer of the endometrium. Am J Epidemiol. 1982 Aug;116(2):333–342. doi: 10.1093/oxfordjournals.aje.a113417. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipton A., Santen R. J., Santner S. J., Harvey H. A., Sanders S. I., Matthews Y. L. Prognostic value of breast cancer aromatase. Cancer. 1992 Oct 1;70(7):1951–1955. doi: 10.1002/1097-0142(19921001)70:7<1951::aid-cncr2820700723>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mahendroo M. S., Means G. D., Mendelson C. R., Simpson E. R. Tissue-specific expression of human P-450AROM. The promoter responsible for expression in adipose tissue is different from that utilized in placenta. J Biol Chem. 1991 Jun 15;266(17):11276–11281. [PubMed] [Google Scholar]
- Mahendroo M. S., Mendelson C. R., Simpson E. R. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J Biol Chem. 1993 Sep 15;268(26):19463–19470. [PubMed] [Google Scholar]
- Mendelson C. R., Corbin C. J., Smith M. E., Smith J., Simpson E. R. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3',5'-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology. 1986 Mar;118(3):968–973. doi: 10.1210/endo-118-3-968. [DOI] [PubMed] [Google Scholar]
- Miller W. R. Aromatase activity in breast tissue. J Steroid Biochem Mol Biol. 1991 Nov;39(5B):783–790. doi: 10.1016/0960-0760(91)90026-2. [DOI] [PubMed] [Google Scholar]
- Naganuma H., Ohtani H., Harada N., Nagura H. Immunoelectron microscopic localization of aromatase in human placenta and ovary using microwave fixation. J Histochem Cytochem. 1990 Oct;38(10):1427–1432. doi: 10.1177/38.10.2401783. [DOI] [PubMed] [Google Scholar]
- Nagasako S., Asanuma N., Nagata Y. [Plasma concentration of estrogens and androgens in postmenopausal women with or without endometrial cancer]. Nihon Sanka Fujinka Gakkai Zasshi. 1988 Jun;40(6):707–713. [PubMed] [Google Scholar]
- Perel E., Wilkins D., Killinger D. W. The conversion of androstenedione to estrone, estradiol, and testosterone in breast tissue. J Steroid Biochem. 1980 Jan;13(1):89–94. doi: 10.1016/0022-4731(80)90117-x. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Singh A., Ghilchik M. W., Coldham N. G., Purohit A. Regulation of oestradiol 17 beta hydroxysteroid dehydrogenase in breast tissues: the role of growth factors. J Steroid Biochem Mol Biol. 1991 Nov;39(5B):791–798. doi: 10.1016/0960-0760(91)90027-3. [DOI] [PubMed] [Google Scholar]
- Sasano H., Nagura H., Harada N., Goukon Y., Kimura M. Immunolocalization of aromatase and other steroidogenic enzymes in human breast disorders. Hum Pathol. 1994 May;25(5):530–535. doi: 10.1016/0046-8177(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Sasano H., Nakashima N., Matsuzaki O., Kato H., Aizawa S., Sasano N., Nagura H. Testicular sex cord-stromal lesions: immunohistochemical analysis of cytokeratin, vimentin and steroidogenic enzymes. Virchows Arch A Pathol Anat Histopathol. 1992;421(2):163–169. doi: 10.1007/BF01607050. [DOI] [PubMed] [Google Scholar]
- Sasano H., Okamoto M., Mason J. I., Simpson E. R., Mendelson C. R., Sasano N., Silverberg S. G. Immunohistochemical studies of steroidogenic enzymes (aromatase, 17 alpha-hydroxylase and cholesterol side-chain cleavage cytochromes P-450) in sex cord-stromal tumors of the ovary. Hum Pathol. 1989 May;20(5):452–457. doi: 10.1016/0046-8177(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Schenker J. G., Weinstein D., Okon E. Estradiol and testosterone levels in the peripheral and ovarian circulations in patients with endometrial cancer. Cancer. 1979 Nov;44(5):1809–1812. doi: 10.1002/1097-0142(197911)44:5<1809::aid-cncr2820440540>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Schweikert H. U., Milewich L., Wilson J. D. Aromatization of androstenedione by cultured human fibroblasts. J Clin Endocrinol Metab. 1976 Oct;43(4):785–795. doi: 10.1210/jcem-43-4-785. [DOI] [PubMed] [Google Scholar]
- Silverberg S. G., Mullen D., Faraci J. A., Makowski E. L., Miller A., Finch J. L., Sutherland J. V. Endometrial carcinoma: clinical-pathologic comparison of cases in postmenopausal women receiving and not receiving exogenous estrogens. Cancer. 1980 Jun 15;45(12):3018–3026. doi: 10.1002/1097-0142(19800615)45:12<3018::aid-cncr2820451224>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sasano H., Sasaki H., Fukaya T., Nagura H. Quantitation of P450 aromatase immunoreactivity in human ovary during the menstrual cycle: relationship between the enzyme activity and immunointensity. J Histochem Cytochem. 1994 Dec;42(12):1565–1573. doi: 10.1177/42.12.7983357. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sasano H., Tamura M., Aoki H., Fukaya T., Yajima A., Nagura H., Mason J. I. Temporal and spatial localization of steroidogenic enzymes in premenopausal human ovaries: in situ hybridization and immunohistochemical study. Mol Cell Endocrinol. 1993 Nov;97(1-2):135–143. doi: 10.1016/0303-7207(93)90220-e. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Jr, Siiteri P. K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem. 1974 Sep 10;249(17):5364–5372. [PubMed] [Google Scholar]
- Tseng L., Mazella J., Mann W. J., Chumas J. Estrogen synthesis in normal and malignant human endometrium. J Clin Endocrinol Metab. 1982 Nov;55(5):1029–1031. doi: 10.1210/jcem-55-5-1029. [DOI] [PubMed] [Google Scholar]
- Yamaki J., Yamamoto T., Okada H. Aromatization of androstenedione by normal and neoplastic endometrium of the uterus. J Steroid Biochem. 1985 Jan;22(1):63–66. doi: 10.1016/0022-4731(85)90142-6. [DOI] [PubMed] [Google Scholar]