Abstract
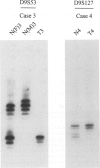
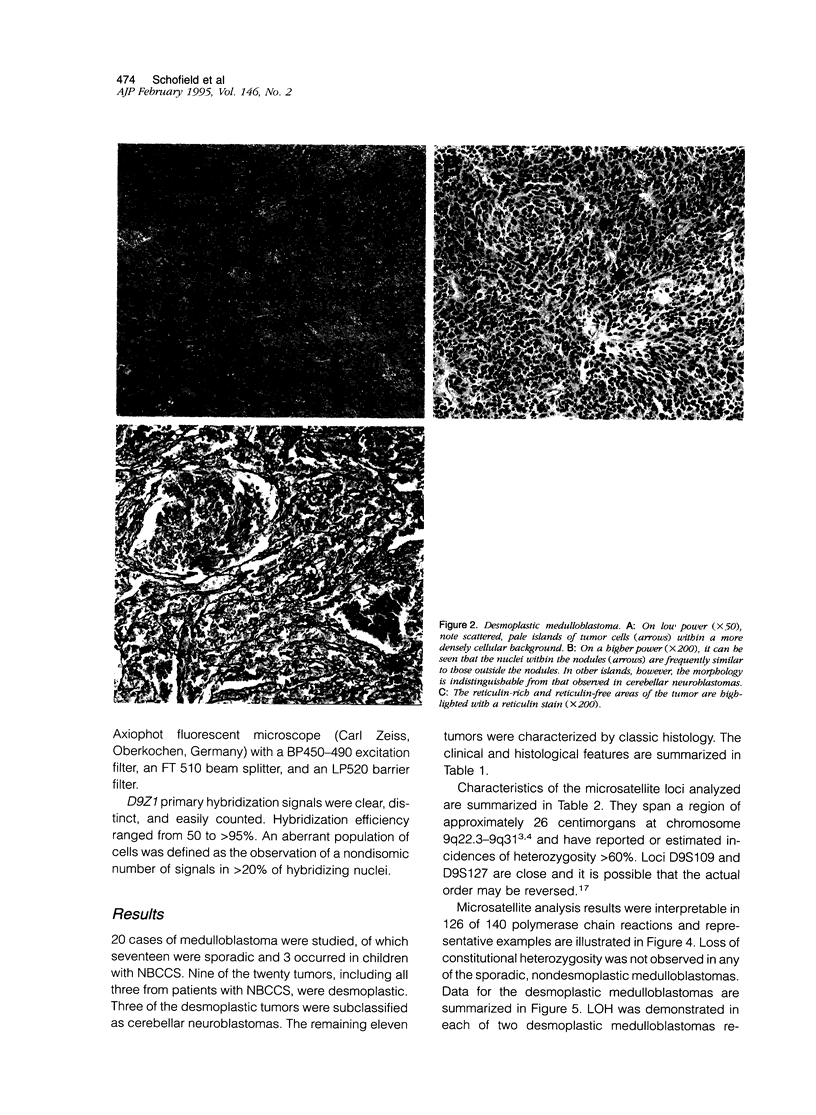
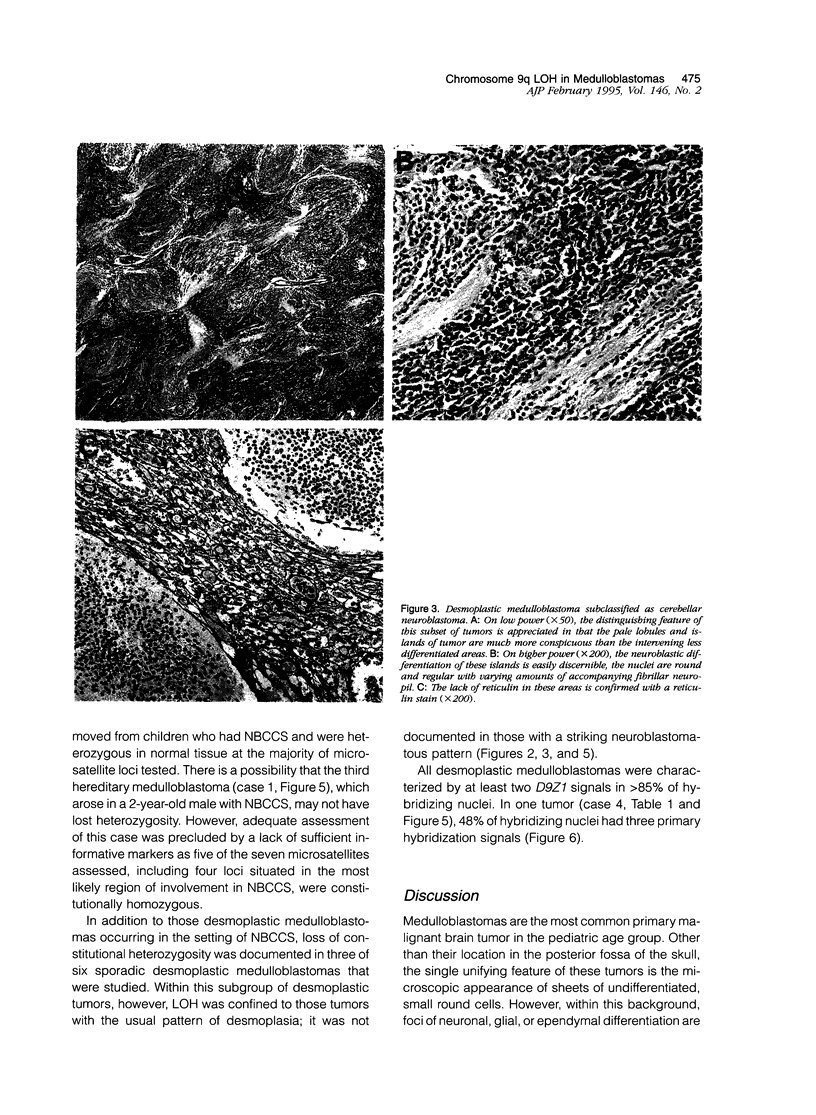
Patients with the nevoid basal cell carcinoma syndrome (NBCCS) are at increased risk for medulloblastomas as well as for basal cell carcinomas. The gene for NBCCS has been mapped to chromosome 9q22.3-q31 by linkage analysis, and loss of heterozygosity (LOH) in this region has been demonstrated in approximately one-half of sporadic basal cell carcinomas. In the present study, LOH for chromosome 9q22.3-q31 microsatellite markers was investigated in medulloblastomas occurring among children with NBCCS and in sporadic medulloblastomas. Histologically, all 3 NBCCS medulloblastomas were noted to have a desmoplastic phenotype, and LOH was detected in both of the 2 cases for which microsatellite markers were heterozygous in normal tissues. LOH was also detected in a subset of sporadic medulloblastomas, each of which were found to display the desmoplastic phenotype. In all, 3 of the 6 sporadic desmoplastic tumors showed LOH, whereas LOH was not seen in any of the 11 sporadic, non-desmoplastic medulloblastomas studied. Additionally, desmoplastic tumors lacking detectable LOH each showed histological features of so-called cerebellar neuroblastoma, a subgroup of desmoplastic medulloblastoma with extensive neuroblastomatous differentiation. The data are consistent with a role for inactivation of a tumor suppressor gene at chromosome 9q in the development of medulloblastomas in patients with NBCCS and of sporadic medulloblastomas characterized by a desmoplastic phenotype similar to that found in patients with NBCCS. Restriction of chromosome 9q loss to non-neuroblastomatous desmoplastic tumors suggests that this variant of medulloblastoma maybe pathogenetically distinct from tumors having other histological phenotypes.
Full text
PDF

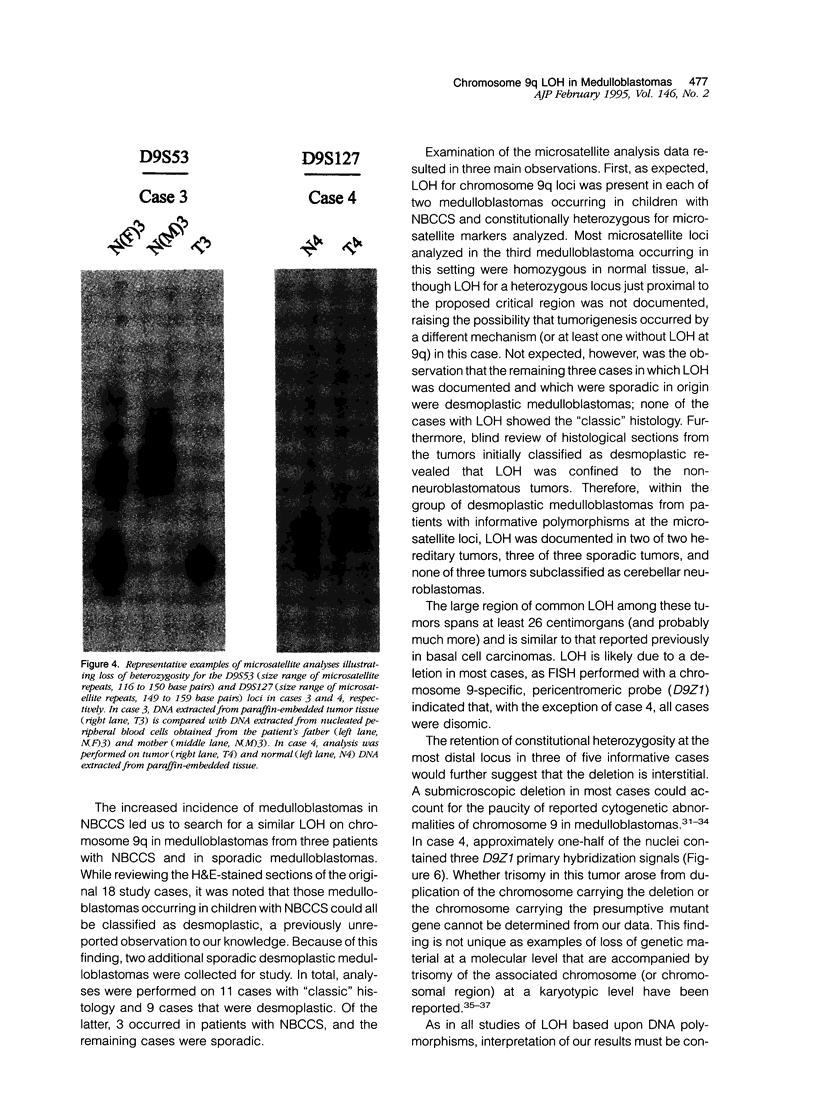
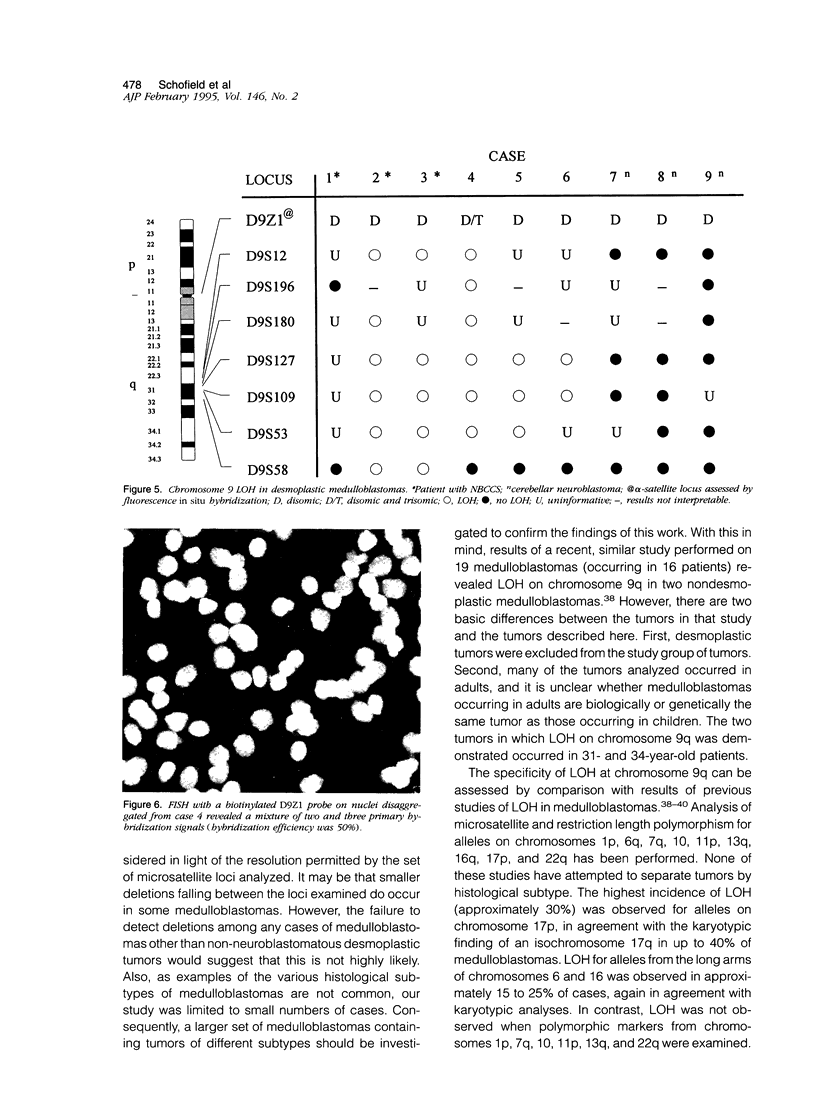


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S., von Deimling A., Pietsch T., Giangaspero F., Brandner S., Kleihues P., Wiestler O. D. Microsatellite analysis of loss of heterozygosity on chromosomes 9q, 11p and 17p in medulloblastomas. Neuropathol Appl Neurobiol. 1994 Feb;20(1):74–81. doi: 10.1111/j.1365-2990.1994.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Biegel J. A., Rorke L. B., Packer R. J., Sutton L. N., Schut L., Bonner K., Emanuel B. S. Isochromosome 17q in primitive neuroectodermal tumors of the central nervous system. Genes Chromosomes Cancer. 1989 Nov;1(2):139–147. doi: 10.1002/gcc.2870010206. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Friedman H. S., Biegel J. A., Bigner D. D. Structural chromosomal abnormalities in human medulloblastoma. Cancer Genet Cytogenet. 1988 Jan;30(1):91–101. doi: 10.1016/0165-4608(88)90096-9. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Cultured diploid fibroblasts from patients with the nevoid basal cell carcinoma syndrome are hypersensitive to killing by ionizing radiation. Am J Pathol. 1983 Apr;111(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- Chatty E. M., Earle K. M. Medulloblastoma. A report of 201 cases with emphasis on the relationship of histologic variants to survival. Cancer. 1971 Oct;28(4):977–983. doi: 10.1002/1097-0142(1971)28:4<977::aid-cncr2820280422>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Chenevix-Trench G., Wicking C., Berkman J., Sharpe H., Hockey A., Haan E., Oley C., Ravine D., Turner A., Goldgar D. Further localization of the gene for nevoid basal cell carcinoma syndrome (NBCCS) in 15 Australasian families: linkage and loss of heterozygosity. Am J Hum Genet. 1993 Sep;53(3):760–767. [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Farndon P. A., Burnell L. D., Gattamaneni H. R., Birch J. M. The incidence of Gorlin syndrome in 173 consecutive cases of medulloblastoma. Br J Cancer. 1991 Nov;64(5):959–961. doi: 10.1038/bjc.1991.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndon P. A., Del Mastro R. G., Evans D. G., Kilpatrick M. W. Location of gene for Gorlin syndrome. Lancet. 1992 Mar 7;339(8793):581–582. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- Furlong R. A., Lyall J. E., Goudie D. R., Leversha M. A., Affara N. A., Ferguson-Smith M. A. A dinucleotide repeat polymorphism at the D9S109 locus. Nucleic Acids Res. 1992 Feb 25;20(4):925–925. doi: 10.1093/nar/20.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani M. R., Bale S. J., Leffell D. J., DiGiovanna J. J., Peck G. L., Poliak S., Drum M. A., Pastakia B., McBride O. W., Kase R. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992 Apr 3;69(1):111–117. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- Geyer J. R., Schofield D., Berger M., Milstein J. Differentiation of a primitive neuroectodermal tumor into a benign ganglioglioma. J Neurooncol. 1992 Nov;14(3):237–241. doi: 10.1007/BF00172599. [DOI] [PubMed] [Google Scholar]
- Giangaspero F., Chieco P., Ceccarelli C., Lisignoli G., Pozzuoli R., Gambacorta M., Rossi G., Burger P. C. "Desmoplastic" versus "classic" medulloblastoma: comparison of DNA content, histopathology and differentiation. Virchows Arch A Pathol Anat Histopathol. 1991;418(3):207–214. doi: 10.1007/BF01606058. [DOI] [PubMed] [Google Scholar]
- Goldstein A. M., Stewart C., Bale A. E., Bale S. J., Dean M. Localization of the gene for the nevoid basal cell carcinoma syndrome. Am J Hum Genet. 1994 May;54(5):765–773. [PMC free article] [PubMed] [Google Scholar]
- Gorlin R. J. Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 1987 Mar;66(2):98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Hawkins A. L., Packer R. J., Rorke L. B., Emanuel B. S. Chromosome abnormalities in pediatric brain tumors. Cancer Res. 1988 Jan 1;48(1):175–180. [PubMed] [Google Scholar]
- HERZBERG J. J., WISKEMANN A. [The fifth phakomatosis. Basal cell nevus with hereditary malformation and medulloblastoma]. Dermatologica. 1963;126:106–123. [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Karnes P. S., Tran T. N., Cui M. Y., Raffel C., Gilles F. H., Barranger J. A., Ying K. L. Cytogenetic analysis of 39 pediatric central nervous system tumors. Cancer Genet Cytogenet. 1992 Mar;59(1):12–19. doi: 10.1016/0165-4608(92)90150-7. [DOI] [PubMed] [Google Scholar]
- Katsetos C. D., Liu H. M., Zacks S. I. Immunohistochemical and ultrastructural observations on Homer Wright (neuroblastic) rosettes and the "pale islands" of human cerebellar medulloblastomas. Hum Pathol. 1988 Oct;19(10):1219–1227. doi: 10.1016/s0046-8177(88)80155-2. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Henske E. P., Weimer K., Ozelius L., Gusella J. F., Haines J. Construction of a GT polymorphism map of human 9q. Genomics. 1992 Feb;12(2):229–240. doi: 10.1016/0888-7543(92)90370-8. [DOI] [PubMed] [Google Scholar]
- Levi S., Urbano-Ispizua A., Gill R., Thomas D. M., Gilbertson J., Foster C., Marshall C. J. Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991 Jul 1;51(13):3497–3502. [PubMed] [Google Scholar]
- Lyall J. E., Furlong R. A., Yuille M. A., Goudie D. R., Leversha M. A., Affara N. A., Ferguson-Smith M. A. A dinucleotide repeat polymorphism at the D9S127 locus. Nucleic Acids Res. 1992 Feb 25;20(4):925–925. doi: 10.1093/nar/20.4.925-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoska J. A., Micale M. A., Sakr W. A., Benson P. D., Wolman S. R. Extensive genetic alterations in prostate cancer revealed by dual PCR and FISH analysis. Genes Chromosomes Cancer. 1993 Oct;8(2):88–97. doi: 10.1002/gcc.2870080205. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Kallioniemi A., Kallioniemi O., Chen L., Smith H. S., Pinkel D., Gray J., Waldman F. M. Deletion of chromosome 17p loci in breast cancer cells detected by fluorescence in situ hybridization. Cancer Res. 1992 Jun 15;52(12):3474–3477. [PubMed] [Google Scholar]
- Pearl G. S., Takei Y. Cerebellar "neuroblastoma": nosology as it relates to medulloblastoma. Cancer. 1981 Feb 15;47(4):772–779. doi: 10.1002/1097-0142(19810215)47:4<772::aid-cncr2820470423>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Povey S., Smith M., Haines J., Kwiatkowski D., Fountain J., Bale A., Abbott C., Jackson I., Lawrie M., Hultén M. Report and abstracts of the First International Workshop on Chromosome 9. Held at Girton College Cambridge, UK, 22-24 March, 1992. Ann Hum Genet. 1992 Jul;56(Pt 3):167–182. doi: 10.1111/j.1469-1809.1992.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Raffel C., Gilles F. E., Weinberg K. I. Reduction to homozygosity and gene amplification in central nervous system primitive neuroectodermal tumors of childhood. Cancer Res. 1990 Feb 1;50(3):587–591. [PubMed] [Google Scholar]
- Rogers B. B. Application of the polymerase chain reaction to archival material. Perspect Pediatr Pathol. 1992;16:99–119. [PubMed] [Google Scholar]
- Thomas G. A., Raffel C. Loss of heterozygosity on 6q, 16q, and 17p in human central nervous system primitive neuroectodermal tumors. Cancer Res. 1991 Jan 15;51(2):639–643. [PubMed] [Google Scholar]
- Warzok R., Jaenisch W., Lang G. Morphology and biology of cerebellar neuroblastomas. J Neurooncol. 1983;1(4):373–379. doi: 10.1007/BF00165721. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilkie P. J., Krizman D. B., Weber J. L. Linkage map of human chromosome 9 microsatellite polymorphisms. Genomics. 1992 Mar;12(3):607–609. doi: 10.1016/0888-7543(92)90456-3. [DOI] [PubMed] [Google Scholar]
- Yagishita S., Itoh Y., Chiba Y., Kuwana N. Morphological investigations on cerebellar "neuroblastoma" group. Acta Neuropathol. 1982;56(1):22–28. doi: 10.1007/BF00691178. [DOI] [PubMed] [Google Scholar]
- Yuille M. A., Leversha M., Goudie D. R., Affara N. A., Ferguson-Smith M. A. Microsatellite polymorphism at the D9S12 locus. Nucleic Acids Res. 1991 Sep 25;19(18):5097–5097. doi: 10.1093/nar/19.18.5097-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chadarévian J. P., Montes J. L., O'Gorman A. M., Freeman C. R. Maturation of cerebellar neuroblastoma into ganglioneuroma with melanosis. A histologic, immunocytochemical, and ultrastructural study. Cancer. 1987 Jan 1;59(1):69–76. doi: 10.1002/1097-0142(19870101)59:1<69::aid-cncr2820590117>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]