Abstract
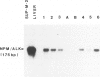
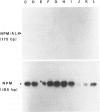
Although cytogenetic data suggest that the t(2;5)-(p23;q35) translocation occurs in many cases of CD30+ lymphomas, the exact frequency of this event is still unknown. To clarify this issue and its epidemiological characteristics, we examined 37 formalin-fixed, paraffin-embedded specimens of CD30+ lymphomas from the United States and Hong Kong by reverse transcriptase-polymerase chain reaction (RT-PCR) for the status of the NPM and ALK genes, which are typically juxtaposed by the t(2;5) translocation. Thirty-four cases were classified as anaplastic large cell lymphomas (ALCL), 2 cases as non-anaplastic large cell lymphomas (LCL), and 1 case as the small cell variant of CD30+ lymphoma. The t(2;5) translocation was detected in 6 cases (16%), including 3 of 18 American patients and 3 of 19 cases from Hong Kong. All cases had a 185-bp NPM RT-PCR product as detected by Southern blot analysis, indicating adequate preservation of mRNA. The 6 positive cases were among 4 of 34 adult lymphomas, as compared with 2 of 3 childhood cases. Five of 17 T-lineage cases were t(2;5)-positive, compared with 1 of 15 B-lineage cases and none of the 5 null-cell or mixed lineage cases. Our results therefore show that t(2;5) occurs at a low frequency among CD30+ lymphomas, at least in our adult-dominated series.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz-Lemoine E., Brizard A., Huret J. L., Babin P., Guilhot F., Couet D., Tanzer J. Malignant histiocytosis: a specific t(2;5)(p23;q35) translocation? Review of the literature. Blood. 1988 Sep;72(3):1045–1047. [PubMed] [Google Scholar]
- Bitter M. A., Franklin W. A., Larson R. A., McKeithan T. W., Rubin C. M., Le Beau M. M., Stephens J. K., Vardiman J. W. Morphology in Ki-1(CD30)-positive non-Hodgkin's lymphoma is correlated with clinical features and the presence of a unique chromosomal abnormality, t(2;5)(p23;q35). Am J Surg Pathol. 1990 Apr;14(4):305–316. doi: 10.1097/00000478-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Bloomfield C. D., Arthur D. C., Frizzera G., Levine E. G., Peterson B. A., Gajl-Peczalska K. J. Nonrandom chromosome abnormalities in lymphoma. Cancer Res. 1983 Jun;43(6):2975–2984. [PubMed] [Google Scholar]
- Bullrich F., Morris S. W., Hummel M., Pileri S., Stein H., Croce C. M. Nucleophosmin (NPM) gene rearrangements in Ki-1-positive lymphomas. Cancer Res. 1994 Jun 1;54(11):2873–2877. [PubMed] [Google Scholar]
- Ebrahim S. A., Ladanyi M., Desai S. B., Offit K., Jhanwar S. C., Filippa D. A., Lieberman P. H., Chaganti R. S. Immunohistochemical, molecular, and cytogenetic analysis of a consecutive series of 20 peripheral T-cell lymphomas and lymphomas of uncertain lineage, including 12 Ki-1 positive lymphomas. Genes Chromosomes Cancer. 1990 May;2(1):27–35. doi: 10.1002/gcc.2870020106. [DOI] [PubMed] [Google Scholar]
- Fischer P., Nacheva E., Mason D. Y., Sherrington P. D., Hoyle C., Hayhoe F. G., Karpas A. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988 Jul;72(1):234–240. [PubMed] [Google Scholar]
- Gordon B. G., Weisenburger D. D., Warkentin P. I., Anderson J., Sanger W. G., Bast M., Gnarra D., Vose J. M., Bierman P. J., Armitage J. O. Peripheral T-cell lymphoma in childhood and adolescence. A clinicopathologic study of 22 patients. Cancer. 1993 Jan 1;71(1):257–263. doi: 10.1002/1097-0142(19930101)71:1<257::aid-cncr2820710139>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Greer J. P., Kinney M. C., Collins R. D., Salhany K. E., Wolff S. N., Hainsworth J. D., Flexner J. M., Stein R. S. Clinical features of 31 patients with Ki-1 anaplastic large-cell lymphoma. J Clin Oncol. 1991 Apr;9(4):539–547. doi: 10.1200/JCO.1991.9.4.539. [DOI] [PubMed] [Google Scholar]
- Headington J. T., Roth M. S., Ginsburg D., Lichter A. S., Hyder D., Schnitzer B. T-cell receptor gene rearrangement in regressing atypical histiocytosis. Arch Dermatol. 1987 Sep;123(9):1183–1187. [PubMed] [Google Scholar]
- Isaacson P. G., O'Connor N. T., Spencer J., Bevan D. H., Connolly C. E., Kirkham N., Pollock D. J., Wainscoat J. S., Stein H., Mason D. Y. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985 Sep 28;2(8457):688–691. doi: 10.1016/s0140-6736(85)92930-7. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. Ki-1/CD30+ (anaplastic) large-cell lymphoma: maturation of a clinicopathologic entity with prospects of effective therapy. J Clin Oncol. 1994 May;12(5):884–887. doi: 10.1200/JCO.1994.12.5.884. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Kaneko Y., Frizzera G., Edamura S., Maseki N., Sakurai M., Komada Y., Sakurai M., Tanaka H., Sasaki M., Suchi T. A novel translocation, t(2;5)(p23;q35), in childhood phagocytic large T-cell lymphoma mimicking malignant histiocytosis. Blood. 1989 Feb 15;73(3):806–813. [PubMed] [Google Scholar]
- Kinney M. C., Collins R. D., Greer J. P., Whitlock J. A., Sioutos N., Kadin M. E. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol. 1993 Sep;17(9):859–868. doi: 10.1097/00000478-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Kristoffersson U., Heim S., Heldrup J., Akerman M., Garwicz S., Mitelman F. Cytogenetic studies of childhood non-Hodgkin lymphomas. Hereditas. 1985;103(1):77–84. doi: 10.1111/j.1601-5223.1985.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Bitter M. A., Larson R. A., Doane L. A., Ellis E. D., Franklin W. A., Rubin C. M., Kadin M. E., Vardiman J. W. The t(2;5)(p23;q35): a recurring chromosomal abnormality in Ki-1-positive anaplastic large cell lymphoma. Leukemia. 1989 Dec;3(12):866–870. [PubMed] [Google Scholar]
- Mason D. Y., Bastard C., Rimokh R., Dastugue N., Huret J. L., Kristoffersson U., Magaud J. P., Nezelof C., Tilly H., Vannier J. P. CD30-positive large cell lymphomas ('Ki-1 lymphoma') are associated with a chromosomal translocation involving 5q35. Br J Haematol. 1990 Feb;74(2):161–168. doi: 10.1111/j.1365-2141.1990.tb02560.x. [DOI] [PubMed] [Google Scholar]
- Morgan R., Hecht B. K., Sandberg A. A., Hecht F., Smith S. D. Chromosome 5q35 breakpoint in malignant histiocytosis. N Engl J Med. 1986 May 15;314(20):1322–1322. doi: 10.1056/nejm198605153142016. [DOI] [PubMed] [Google Scholar]
- Morgan R., Smith S. D., Hecht B. K., Christy V., Mellentin J. D., Warnke R., Cleary M. L. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis. Blood. 1989 Jun;73(8):2155–2164. [PubMed] [Google Scholar]
- Morris S. W., Kirstein M. N., Valentine M. B., Dittmer K. G., Shapiro D. N., Saltman D. L., Look A. T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994 Mar 4;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Rimokh R., Magaud J. P., Berger F., Samarut J., Coiffier B., Germain D., Mason D. Y. A translocation involving a specific breakpoint (q35) on chromosome 5 is characteristic of anaplastic large cell lymphoma ('Ki-1 lymphoma'). Br J Haematol. 1989 Jan;71(1):31–36. doi: 10.1111/j.1365-2141.1989.tb06270.x. [DOI] [PubMed] [Google Scholar]
- Sandlund J. T., Pui C. H., Santana V. M., Mahmoud H., Roberts W. M., Morris S., Raimondi S., Ribeiro R., Crist W. M., Lin J. S. Clinical features and treatment outcome for children with CD30+ large-cell non-Hodgkin's lymphoma. J Clin Oncol. 1994 May;12(5):895–898. doi: 10.1200/JCO.1994.12.5.895. [DOI] [PubMed] [Google Scholar]
- Soulie J., Rousseau-Merck M. F., Mouly H., Nezelof C. Cytogenetic study of malignant histiocytosis transplanted into nude mice; presence of translocation between chromosomes 5 and 6 and a unique marker (13q+). Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;50(4):339–344. doi: 10.1007/BF02889912. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Trela M. J., Cleary M. L., Turner R. R., Warnke R. A., Sklar J. Frequent immunoglobulin and T-cell receptor gene rearrangements in "histiocytic" neoplasms. Am J Pathol. 1985 Dec;121(3):369–373. [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Wood G. S., Trela M., Warnke R. A., Sklar J. Clonal T-cell populations in lymphomatoid papulosis. Evidence of a lymphoproliferative origin for a clinically benign disease. N Engl J Med. 1986 Aug 21;315(8):475–479. doi: 10.1056/NEJM198608213150802. [DOI] [PubMed] [Google Scholar]
- Willemze R., Beljaards R. C., Meijer C. J. Classification of primary cutaneous T-cell lymphomas. Histopathology. 1994 May;24(5):405–415. doi: 10.1111/j.1365-2559.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Willemze R., Beljaards R. C. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders. A proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993 Jun;28(6):973–980. doi: 10.1016/0190-9622(93)70140-o. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]