Abstract
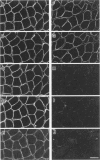
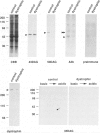
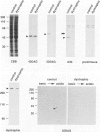
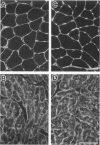
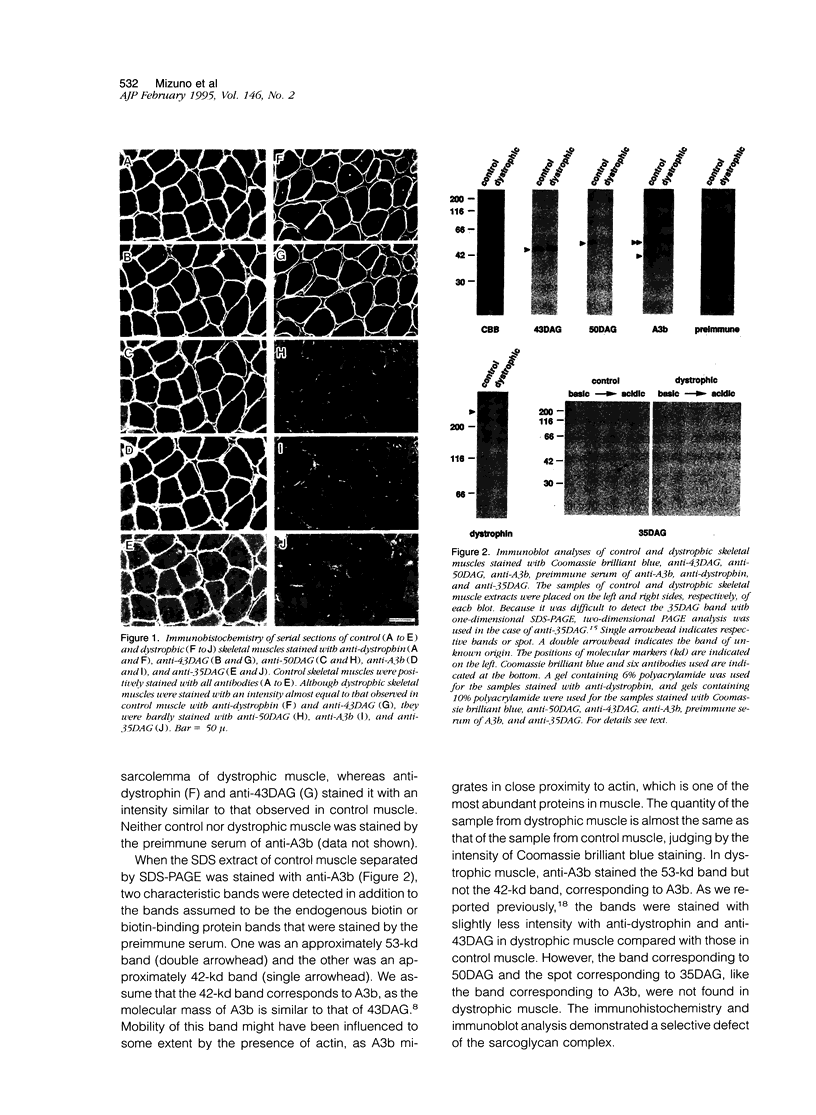
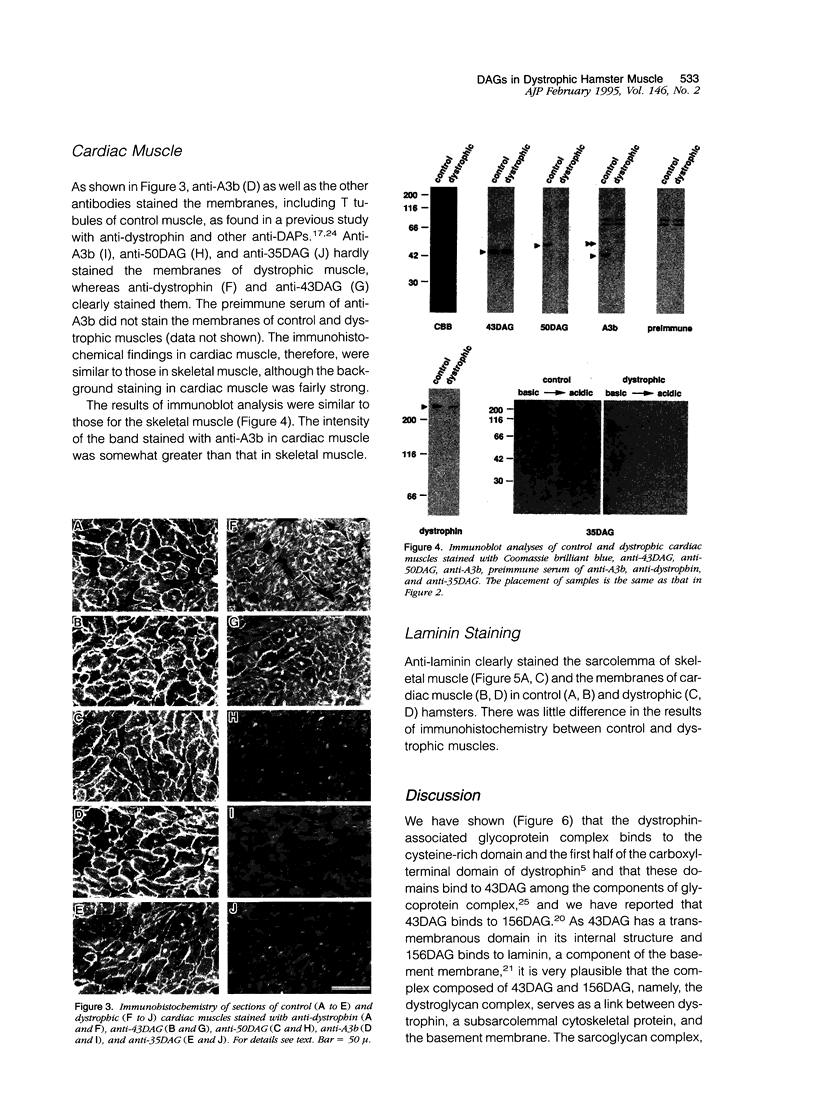
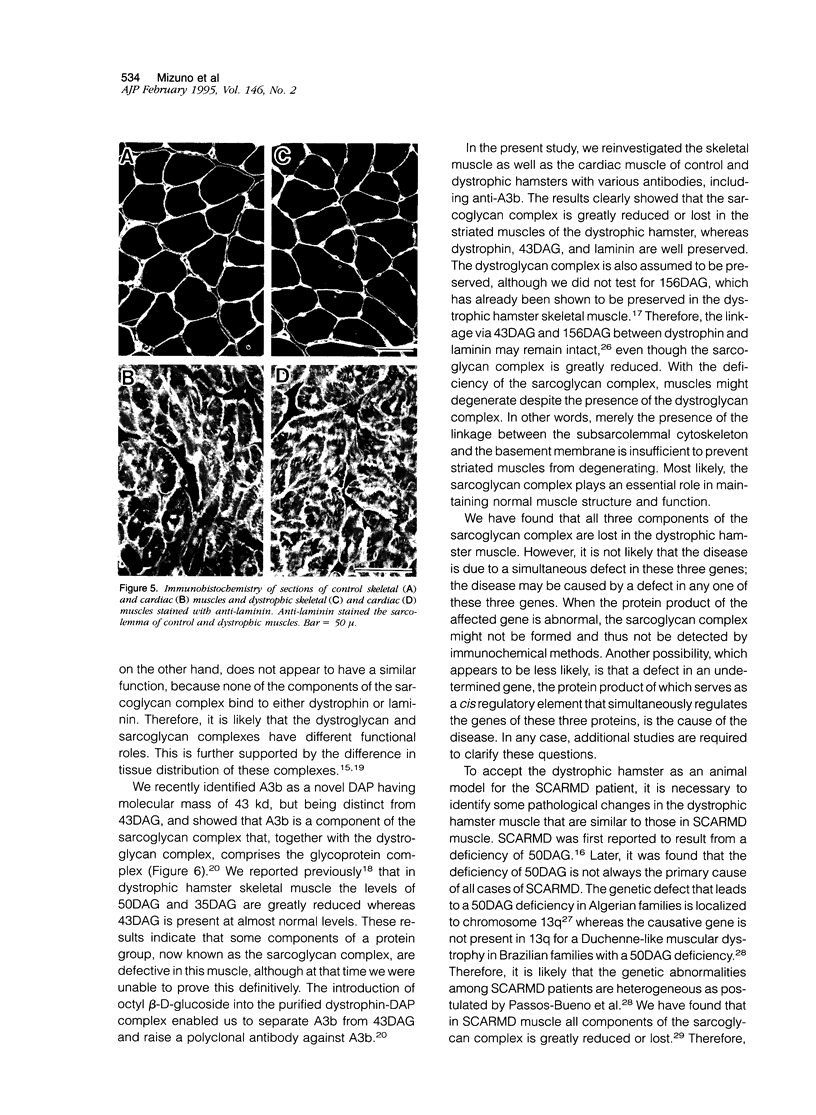
We recently reported that the dystrophin-associated glycoprotein (DAG) complex is biochemically divided into two subcomplexes: one is the dystroglycan complex comprised of 156DAG and 43DAG and the other is the sarcoglycan complex comprised of 50DAG, A3b, and 35DAG. A3b is a novel dystrophin-associated glycoprotein with an approximate molecular mass of 43 kd but is distinct from 43DAG. In the present study, we examined the striated muscles of the dystrophic hamster with anti-A3b antibody in addition to anti-50DAG, anti-43DAG, anti-35DAG, anti-dystrophin, and anti-laminin antibodies by both immunohistochemistry and immunoblot analysis and found that 50DAG, A3b, and 35DAG are selectively lost. This selective defect of the sarcoglycan complex in dystrophic hamster muscles may give rise to dystrophic changes in striated muscles. Thus, the differentiation of the dystrophin-associated glycoprotein complex into the dystroglycan and sarcoglycan complexes is important not only from a biochemical standpoint but also in understanding the cause of muscular dystrophy in the hamster. Our findings further show that the dystrophic hamster may serve as an animal model for a human disease, severe childhood autosomal recessive muscular dystrophy, which has recently been shown to result from a selective defect in the sarcoglycan complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn A. H., Yoshida M., Anderson M. S., Feener C. A., Selig S., Hagiwara Y., Ozawa E., Kunkel L. M. Cloning of human basic A1, a distinct 59-kDa dystrophin-associated protein encoded on chromosome 8q23-24. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4446–4450. doi: 10.1073/pnas.91.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Azibi K., Bachner L., Beckmann J. S., Matsumura K., Hamouda E., Chaouch M., Chaouch A., Ait-Ouarab R., Vignal A., Weissenbach J. Severe childhood autosomal recessive muscular dystrophy with the deficiency of the 50 kDa dystrophin-associated glycoprotein maps to chromosome 13q12. Hum Mol Genet. 1993 Sep;2(9):1423–1428. doi: 10.1093/hmg/2.9.1423. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Iwata Y., Nakamura H., Mizuno Y., Yoshida M., Ozawa E., Shigekawa M. Defective association of dystrophin with sarcolemmal glycoproteins in the cardiomyopathic hamster heart. FEBS Lett. 1993 Aug 23;329(1-2):227–231. doi: 10.1016/0014-5793(93)80227-l. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Noguchi S., Yamamoto H., Yoshida M., Suzuki A., Hagiwara Y., Hayashi Y. K., Arahata K., Nonaka I., Hirai S. Selective defect of sarcoglycan complex in severe childhood autosomal recessive muscular dystrophy muscle. Biochem Biophys Res Commun. 1994 Sep 15;203(2):979–983. doi: 10.1006/bbrc.1994.2278. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Nonaka I., Hirai S., Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993 Oct;119(1):43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Yoshida M., Nonaka I., Hirai S., Ozawa E. Expression of utrophin (dystrophin-related protein) and dystrophin-associated glycoproteins in muscles from patients with Duchenne muscular dystrophy. Muscle Nerve. 1994 Feb;17(2):206–216. doi: 10.1002/mus.880170212. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Yoshida M., Yamamoto H., Hirai S., Ozawa E. Distribution of dystrophin isoforms and dystrophin-associated proteins 43DAG (A3a) and 50DAG (A2) in various monkey tissues. J Biochem. 1993 Dec;114(6):936–941. doi: 10.1093/oxfordjournals.jbchem.a124281. [DOI] [PubMed] [Google Scholar]
- Nicholson L. V., Johnson M. A., Davison K., O'Donnell E., Falkous G., Barron M., Harris J. B. Dystrophin or a "related protein" in Duchenne muscular dystrophy? Acta Neurol Scand. 1992 Jul;86(1):8–14. doi: 10.1111/j.1600-0404.1992.tb08046.x. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V. V., Towbin J. A., Bosch E. P., Weinstein S. L., Sernett S. W., Campbell K. P. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 1993 Apr;43(4):795–800. doi: 10.1212/wnl.43.4.795. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Oliveira J. R., Bakker E., Anderson R. D., Marie S. K., Vainzof M., Roberds S., Campbell K. P., Zatz M. Genetic heterogeneity for Duchenne-like muscular dystrophy (DLMD) based on linkage and 50 DAG analysis. Hum Mol Genet. 1993 Nov;2(11):1945–1947. doi: 10.1093/hmg/2.11.1945. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Ervasti J. M., Anderson R. D., Ohlendieck K., Kahl S. D., Zoloto D., Campbell K. P. Disruption of the dystrophin-glycoprotein complex in the cardiomyopathic hamster. J Biol Chem. 1993 Jun 5;268(16):11496–11499. [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Hayashi K., Mizuno Y., Hagiwara Y., Ozawa E. Molecular organization at the glycoprotein-complex-binding site of dystrophin. Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur J Biochem. 1994 Mar 1;220(2):283–292. doi: 10.1111/j.1432-1033.1994.tb18624.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Yamamoto H., Ozawa E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 1992 Aug 17;308(2):154–160. doi: 10.1016/0014-5793(92)81265-n. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Way M., Pope B., Cross R. A., Kendrick-Jones J., Weeds A. G. Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett. 1992 Apr 27;301(3):243–245. doi: 10.1016/0014-5793(92)80249-g. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hagiwara Y., Mizuno Y., Yoshida M., Ozawa E. Heterogeneity of dystrophin-associated proteins. J Biochem. 1993 Jul;114(1):132–139. doi: 10.1093/oxfordjournals.jbchem.a124128. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Mizuno Y., Hayashi K., Nonaka I., Yoshida M., Ozawa E. Expression of dystrophin-associated protein 35DAG (A4) and 50DAG (A2) is confined to striated muscles. J Biochem. 1994 Jan;115(1):162–167. doi: 10.1093/oxfordjournals.jbchem.a124294. [DOI] [PubMed] [Google Scholar]
- Yamanouchi Y., Mizuno Y., Yamamoto H., Takemitsu M., Yoshida M., Nonaka I., Ozawa E. Selective defect in dystrophin-associated glycoproteins 50DAG (A2) and 35DAG (A4) in the dystrophic hamster: an animal model for severe childhood autosomal recessive muscular dystrophy (SCARMD). Neuromuscul Disord. 1994 Jan;4(1):49–54. doi: 10.1016/0960-8966(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Yang B., Ibraghimov-Beskrovnaya O., Moomaw C. R., Slaughter C. A., Campbell K. P. Heterogeneity of the 59-kDa dystrophin-associated protein revealed by cDNA cloning and expression. J Biol Chem. 1994 Feb 25;269(8):6040–6044. [PubMed] [Google Scholar]
- Yoshida M., Mizuno Y., Nonaka I., Ozawa E. A dystrophin-associated glycoprotein, A3a (one of 43DAG doublets), is retained in Duchenne muscular dystrophy muscle. J Biochem. 1993 Nov;114(5):634–639. doi: 10.1093/oxfordjournals.jbchem.a124229. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990 Nov;108(5):748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Suzuki A., Yamamoto H., Noguchi S., Mizuno Y., Ozawa E. Dissociation of the complex of dystrophin and its associated proteins into several unique groups by n-octyl beta-D-glucoside. Eur J Biochem. 1994 Jun 15;222(3):1055–1061. doi: 10.1111/j.1432-1033.1994.tb18958.x. [DOI] [PubMed] [Google Scholar]