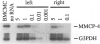Abstract
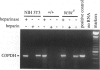
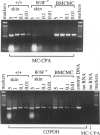
The reverse transcriptase polymerase chain reaction (RT-PCR) procedure is markedly inhibited in specimens of blood that contain commercial heparin as an anticoagulant or in cell preparations containing rat or mouse peritoneal mast cells. However, it was not known whether the levels of endogenous, mast cell-associated heparin that are present in some mammalian tissues are sufficient to interfere with the use of RT-PCR in these settings. We show that RT-PCR detects little or no mRNA transcripts for either mast cell-associated products, such as mouse mast cell-associated protease-2 or -4 (MMCP-2 or MMCP-4) or mast cell carboxypeptidase A, or for mast cell-nonspecific products, such as glyceraldehyde 3-phosphate dehydrogenase, in routinely prepared specimens of cells or tissues that include populations of heparin-containing mast cells. However, signals for mast cell-associated or mast cell-nonspecific transcripts can be readily detected in such specimens if they are treated with heparinase before RT-PCR. RT-PCR after heparinase treatment appears to represent an extremely sensitive method for detecting mast cell-associated transcripts in tissue specimens, permitting the identification of transcripts for mast cell-specific proteases in the skin of genetically mast cell-deficient WBB6F1-W/WV mice, a tissue that contains few or no mast cells according to histological analysis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Gelbart T., Kuhl W. Interference of heparin with the polymerase chain reaction. Biotechniques. 1990 Aug;9(2):166–166. [PubMed] [Google Scholar]
- Brennessel B. A., Buhrer D. P., Gottlieb A. A. Use of insoluble heparin for isolation of DNA polymerase enzymes from murine myeloma. Anal Biochem. 1978 Jul 1;87(2):411–417. doi: 10.1016/0003-2697(78)90690-5. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Srivastava B. I. Inhibition of deoxynucleotide-polymerizing enzyme activities of human cells and of simian sarcoma virus by heparin. Cancer Res. 1978 Aug;38(8):2401–2407. [PubMed] [Google Scholar]
- Ebi Y., Kanakura Y., Jippo-Kanemoto T., Tsujimura T., Furitsu T., Ikeda H., Adachi S., Kasugai T., Nomura S., Kanayama Y. Low c-kit expression of cultured mast cells of mi/mi genotype may be involved in their defective responses to fibroblasts that express the ligand for c-kit. Blood. 1992 Sep 15;80(6):1454–1462. [PubMed] [Google Scholar]
- Eklund K. K., Ghildyal N., Austen K. F., Friend D. S., Schiller V., Stevens R. L. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J Exp Med. 1994 Jul 1;180(1):67–73. doi: 10.1084/jem.180.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Bhavanandan V. P. Influences of anionic polysaccharides on DNA synthesis in isolated nuclei and by DNA polymerase alpha: correlation of observed effects with properties of the polysaccharides. Biochim Biophys Acta. 1983 Sep 9;740(4):466–475. doi: 10.1016/0167-4781(83)90096-9. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New insights into "the riddle of the mast cells": microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990 Jan;62(1):5–33. [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991 Jul 1;174(1):103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodniy M., Kim S., Katzenstein D., Konrad M., Groves E., Merigan T. C. Inhibition of human immunodeficiency virus gene amplification by heparin. J Clin Microbiol. 1991 Apr;29(4):676–679. doi: 10.1128/jcm.29.4.676-679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izraeli S., Pfleiderer C., Lion T. Detection of gene expression by PCR amplification of RNA derived from frozen heparinized whole blood. Nucleic Acids Res. 1991 Nov 11;19(21):6051–6051. doi: 10.1093/nar/19.21.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Fritze L., Galli S. J., Karp G., Rosenberg R. D. Microvascular heparin-like species with anticoagulant activity. Am J Physiol. 1983 Nov;245(5 Pt 1):H725–H733. doi: 10.1152/ajpheart.1983.245.5.H725. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Galli S. J., Jackman R. W., Rosenberg R. D. Anticoagulantly active heparin-like molecules from mast cell-deficient mice. Am J Physiol. 1986 May;250(5 Pt 2):H879–H888. doi: 10.1152/ajpheart.1986.250.5.H879. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Nagao K., Yokoro K., Aaronson S. A. Continuous lines of basophil/mast cells derived from normal mouse bone marrow. Science. 1981 Apr 17;212(4492):333–335. doi: 10.1126/science.7209531. [DOI] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. Immunoaffinity-purified DNA polymerase alpha displays novel properties. Biochemistry. 1987 Dec 15;26(25):8458–8466. doi: 10.1021/bi00399a064. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Balan V., Fernandez-Badilla C. L., Fernandez J. M. RT-PCR cloning of Rab3 isoforms expressed in peritoneal mast cells. FEBS Lett. 1994 Feb 14;339(1-2):171–174. doi: 10.1016/0014-5793(94)80409-5. [DOI] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H. R., Austen K. F., Stevens R. L., Galli S. J., Kitamura Y. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med. 1987 Mar 1;165(3):615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Reynolds D. S., Serafin W. E., Faller D. V., Wall D. A., Abbas A. K., Dvorak A. M., Austen K. F., Stevens R. L. Immortalization of murine connective tissue-type mast cells at multiple stages of their differentiation by coculture of splenocytes with fibroblasts that produce Kirsten sarcoma virus. J Biol Chem. 1988 Sep 5;263(25):12783–12791. [PubMed] [Google Scholar]
- Reynolds D. S., Stevens R. L., Gurley D. S., Lane W. S., Austen K. F., Serafin W. E. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J Biol Chem. 1989 Nov 25;264(33):20094–20099. [PubMed] [Google Scholar]
- Serafin W. E., Reynolds D. S., Rogelj S., Lane W. S., Conder G. A., Johnson S. S., Austen K. F., Stevens R. L. Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J Biol Chem. 1990 Jan 5;265(1):423–429. [PubMed] [Google Scholar]
- Serafin W. E., Sullivan T. P., Conder G. A., Ebrahimi A., Marcham P., Johnson S. S., Austen K. F., Reynolds D. S. Cloning of the cDNA and gene for mouse mast cell protease 4. Demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J Biol Chem. 1991 Jan 25;266(3):1934–1941. [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989 Nov;10(11):381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Friend D. S., McNeil H. P., Schiller V., Ghildyal N., Austen K. F. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Wang T. H., Sheu J. C., Lin S. M., Lin J. T., Chen D. S. Effects of anticoagulants and storage of blood samples on efficacy of the polymerase chain reaction assay for hepatitis C virus. J Clin Microbiol. 1992 Mar;30(3):750–753. doi: 10.1128/jcm.30.3.750-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wershil B. K., Mekori Y. A., Murakami T., Galli S. J. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987 Oct 15;139(8):2605–2614. [PubMed] [Google Scholar]
- Yano H., Wershil B. K., Arizono N., Galli S. J. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989 Oct;84(4):1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]