Abstract
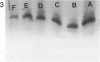
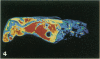
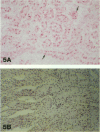
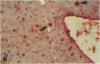
Antisense oligonucleotides have the ability to inhibit individual gene expression in the potential treatment of cancer and viral diseases. However, the way parenterally administered oligonucleotides distribute themselves into healthy tissues or tumors is poorly understood. In this study, the cell and tissue distribution of two modified or unmodified phosphodiester pentadeca-beta-oligonucleotides intravenously administered to healthy or tumor-bearing nude mice was assessed by autoradiography as well as by direct fluorescence and immunoenzymatic histological methods. Resistance of oligonucleotides to degradation by nuclease activity was previously studied in vitro. Using these methods we were able to show the following: 1) within minutes, oligonucleotides permeate all cells and tissues with the exceptions of erythrocytes and intervertebral discs; 2) cell and tissue distribution does not depend on the sequence of the given oligonucleotide; 3) concentration of oligonucleotides is higher within the connective tissue cells than in the interstitial matrix; 4) after uptake, oligomers partition throughout all of the cellular compartments, including at the highest intracellular concentrations in the nuclei; 5) oligonucleotides penetrate easily the tumor cell compartments, oligonucleotide diffusion being unimpeded by the extracellular matrix.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Temsamani J., Tang J. Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon T. A., Morvan F., Rayner B., Imbach J. L., Wickstrom E. alpha-Oligodeoxynucleotide stability in serum, subcellular extracts and culture media. J Biochem Biophys Methods. 1988 Aug;16(4):311–318. doi: 10.1016/0165-022x(88)90065-6. [DOI] [PubMed] [Google Scholar]
- Bacon T. A., Wickstrom E. Walking along human c-myc mRNA with antisense oligodeoxynucleotides: maximum efficacy at the 5' cap region. Oncogene Res. 1991;6(1):13–19. [PubMed] [Google Scholar]
- Bergan R., Connell Y., Fahmy B., Neckers L. Electroporation enhances c-myc antisense oligodeoxynucleotide efficacy. Nucleic Acids Res. 1993 Jul 25;21(15):3567–3573. doi: 10.1093/nar/21.15.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G., Lemoine N. R. Antisense technology for cancer therapy: does it make sense? Br J Cancer. 1993 May;67(5):869–876. doi: 10.1038/bjc.1993.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L., Miller P. S., Ts'o P. O., Colvin O. M., Chem T. L. Disposition and metabolism of oligodeoxynucleoside methylphosphonate following a single i.v. injection in mice. Drug Metab Dispos. 1990 Sep-Oct;18(5):815–818. [PubMed] [Google Scholar]
- Chiasson B. J., Hooper M. L., Murphy P. R., Robertson H. A. Antisense oligonucleotide eliminates in vivo expression of c-fos in mammalian brain. Eur J Pharmacol. 1992 Dec 1;227(4):451–453. doi: 10.1016/0922-4106(92)90167-t. [DOI] [PubMed] [Google Scholar]
- Duprez A., Guerret S., Vignaud J. M., Plenat F., Hartmann D., Grimaud J. A. The interstitial matrix of human carcinomas and sarcomas transplanted to the nude mouse: immunolocalization of some human and murine components. Cell Mol Biol. 1987;33(5):647–654. [PubMed] [Google Scholar]
- Dvorak H. F., Nagy J. A., Dvorak A. M. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells. 1991 Mar;3(3):77–85. [PubMed] [Google Scholar]
- Eder P. S., DeVine R. J., Dagle J. M., Walder J. A. Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3' exonuclease in plasma. Antisense Res Dev. 1991 Summer;1(2):141–151. doi: 10.1089/ard.1991.1.141. [DOI] [PubMed] [Google Scholar]
- Emlen W., Mannik M. Effect of DNA size and strandedness on the in vivo clearance and organ localization of DNA. Clin Exp Immunol. 1984 Apr;56(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990 Nov;9(3):253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay T., Tainsky M., Cavender A. C., Roth J. A. Specific inhibition of K-ras expression and tumorigenicity of lung cancer cells by antisense RNA. Cancer Res. 1991 Mar 15;51(6):1744–1748. [PubMed] [Google Scholar]
- Offensperger W. B., Offensperger S., Walter E., Teubner K., Igloi G., Blum H. E., Gerok W. In vivo inhibition of duck hepatitis B virus replication and gene expression by phosphorothioate modified antisense oligodeoxynucleotides. EMBO J. 1993 Mar;12(3):1257–1262. doi: 10.1002/j.1460-2075.1993.tb05767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenat F., Duprez A. Chimérisme tissulaire homme-souris nude: caractérisation des populations cellulaires par hybridation in situ. C R Acad Sci III. 1989;310(3):53–59. [PubMed] [Google Scholar]
- Plenat F., Martinet Y., Martinet N., Vignaud J. M. Immunohistochemical methods for studying mononuclear phagocytes in tissue sections. J Immunol Methods. 1994 Sep 14;174(1-2):133–154. doi: 10.1016/0022-1759(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Akhtar S., Periasamy A., Herman B., Juliano R. L. Mechanism of cellular uptake of modified oligodeoxynucleotides containing methylphosphonate linkages. Nucleic Acids Res. 1991 Oct 25;19(20):5543–5550. doi: 10.1093/nar/19.20.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Tidd D. M. A potential role for antisense oligonucleotide analogues in the development of oncogene targeted cancer chemotherapy. Anticancer Res. 1990 Sep-Oct;10(5A):1169–1182. [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Karamyshev V. N., Yakubov L. A. Penetration of oligonucleotides into mouse organism through mucosa and skin. FEBS Lett. 1993 Aug 2;327(3):271–274. doi: 10.1016/0014-5793(93)81002-h. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Geselowitz D., Chavany C., Fahmy B., Walbridge S., Alger J. R., Neckers L. M. Stability, clearance, and disposition of intraventricularly administered oligodeoxynucleotides: implications for therapeutic application within the central nervous system. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4665–4669. doi: 10.1073/pnas.90.10.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendegui J. G., Vasquez K. M., Tinsley J. H., Kessler D. J., Hogan M. E. In vivo stability and kinetics of absorption and disposition of 3' phosphopropyl amine oligonucleotides. Nucleic Acids Res. 1992 Jan 25;20(2):307–314. doi: 10.1093/nar/20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]