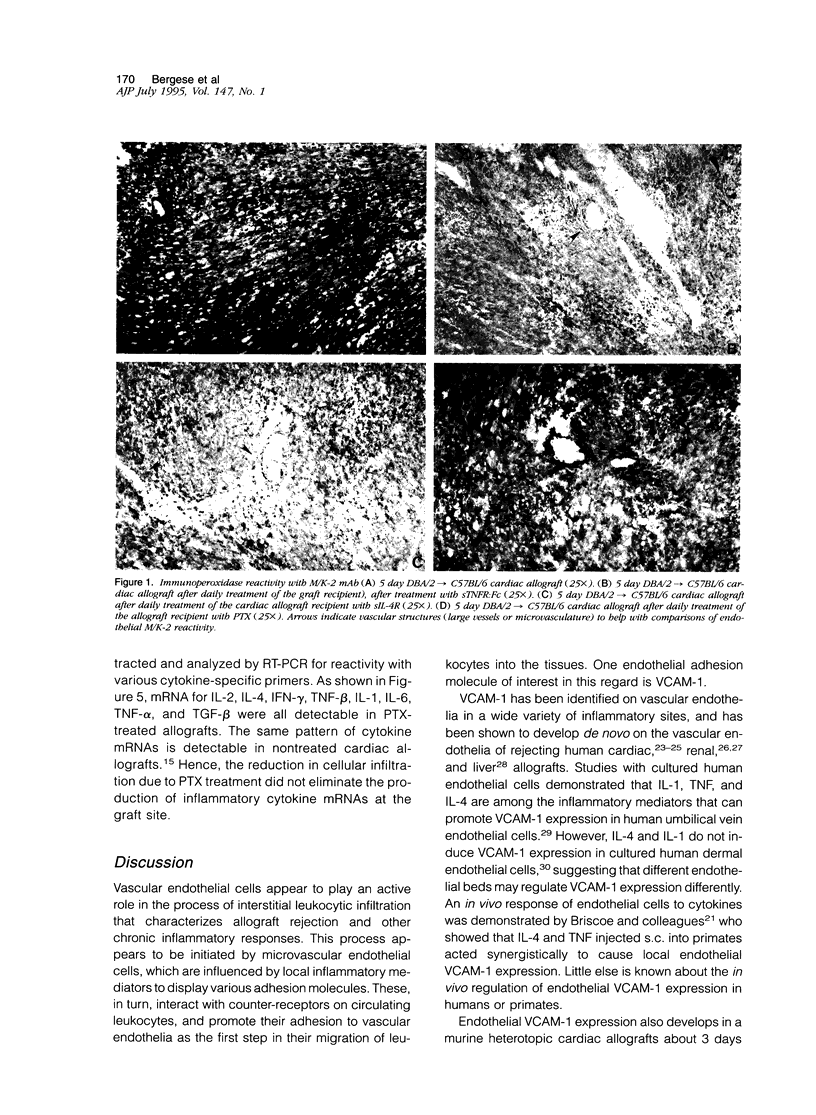
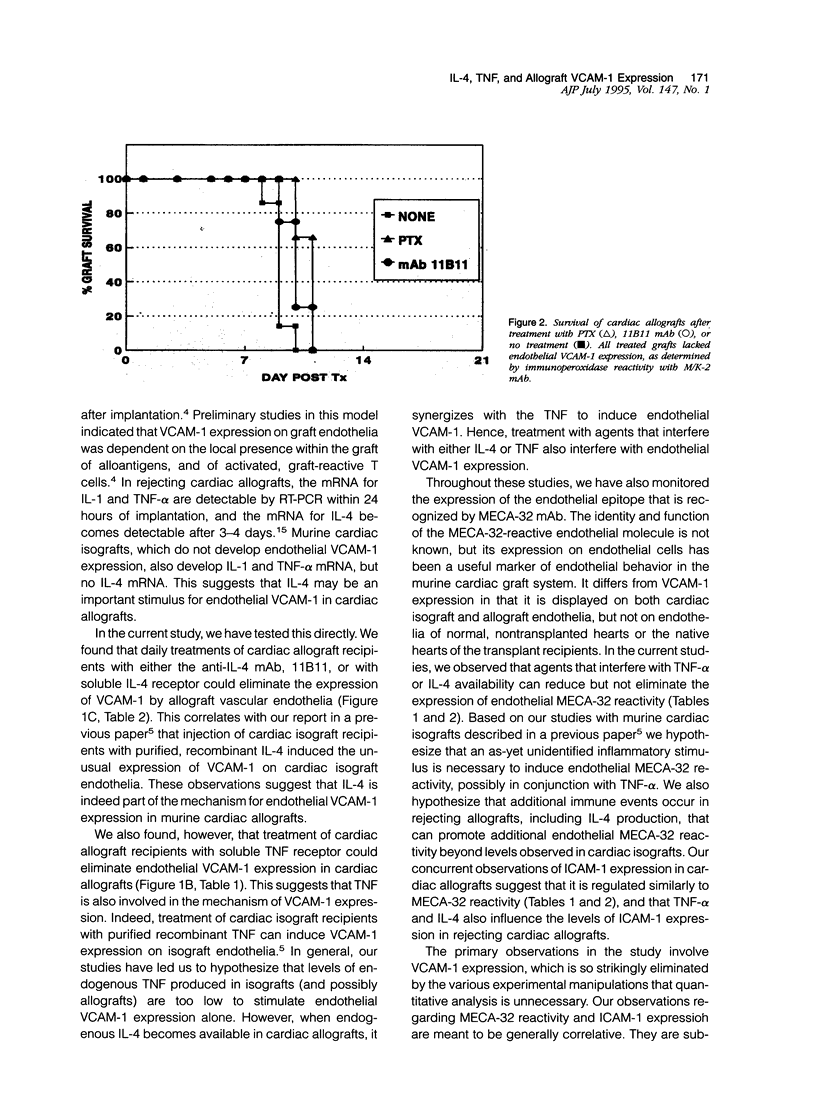
Abstract
This report provides evidence to support the hypothesis that tumor necrosis factor-alpha (TNF-alpha) and IL-4 promote the expression of new endothelial surface molecules in rejecting murine heterotopic cardiac allografts. The microvascular endothelia of these cardiac allografts all develop strong reactivity with the monoclonal antibodies (mAbs) YN1.1/74 (anti-ICAM-1), M/K-2 (anti-VCAM-1) and MECA-32 (undefined molecule) within 3 to 5 days of graft implantation. Daily treatment of the allograft recipients with pentoxifylline (PTX), soluble TNF receptor (TNFR:Fc), anti-interleukin-4 (IL-4) mAb (11B11), or soluble IL-4 receptor, each abrogate the expression of endothelial VCAM-1 and reduce the endothelial reactivity with the mAbs YN1.1/74 and MECA-32 to levels found in cardiac isografts. This is accompanied by a reduction, but not an elimination, of interstitial leukocytic infiltration. Despite this, cardiac allograft recipients treated with PTX or the mAb 11B11 rejected allografts at the same rate as untreated allograft recipients, ie, within 10 to 12 days after graft implantation. These rejected grafts contained mRNAs for TNF-alpha and IL-4, as well as for all other cytokines that have been associated with rejecting allografts. This suggests that endothelial VCAM-1 expression, which is characteristic of rejection, is not an essential element of the rejection process. Interestingly, the grafts from the PTX-treated recipients continued to display rare, isolated VCAM-1 positive cells in the interstitium, which may be dendritic cells. In general, these studies demonstrate a role for IL-4 and TNF-alpha in the alterations of vascular endothelial phenotype observed in rejecting cardiac allografts. They also demonstrate that endothelial VCAM-1 expression is not essential for the allograft rejection process, and that the role of VCAM-1 in this process may be more subtle than was initially suspected.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegre M. L., Gastaldello K., Abramowicz D., Kinnaert P., Vereerstraeten P., De Pauw L., Vandenabeele P., Moser M., Leo O., Goldman M. Evidence that pentoxifylline reduces anti-CD3 monoclonal antibody-induced cytokine release syndrome. Transplantation. 1991 Oct;52(4):674–679. doi: 10.1097/00007890-199110000-00018. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Davis C. L., Marsh C. L., Riches W., McCarty J. M., Benjamin C. D., Carlos T. M., Harlan J. M., Lobb R. Expression of vascular cell adhesion molecule-1 in kidney allograft rejection. Kidney Int. 1993 Oct;44(4):805–816. doi: 10.1038/ki.1993.315. [DOI] [PubMed] [Google Scholar]
- Bacchi C. E., Marsh C. L., Perkins J. D., Carithers R. L., Jr, McVicar J. P., Hudkins K. L., Benjamin C. D., Harlan J. M., Lobb R., Alpers C. E. Expression of vascular cell adhesion molecule (VCAM-1) in liver and pancreas allograft rejection. Am J Pathol. 1993 Feb;142(2):579–591. [PMC free article] [PubMed] [Google Scholar]
- Bergese S. D., Pelletier R. P., Ohye R. G., Vallera D. A., Orosz C. G. Treatment of mice with anti-CD3 mAb induces endothelial vascular cell adhesion molecule-1 expression. Transplantation. 1994 Mar 15;57(5):711–717. doi: 10.1097/00007890-199403150-00013. [DOI] [PubMed] [Google Scholar]
- Bergese S., Pelletier R., Vallera D., Widmer M., Orosz C. Regulation of endothelial VCAM-1 expression in murine cardiac grafts. Roles for TNF and IL4. Am J Pathol. 1995 Apr;146(4):989–998. [PMC free article] [PubMed] [Google Scholar]
- Briscoe D. M., Cotran R. S., Pober J. S. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992 Nov 1;149(9):2954–2960. [PubMed] [Google Scholar]
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Carlos T., Gordon D., Fishbein D., Himes V. E., Coday A., Ross R., Allen M. D. Vascular cell adhesion molecule-1 is induced on endothelium during acute rejection in human cardiac allografts. J Heart Lung Transplant. 1992 Nov-Dec;11(6):1103–1109. [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Heart transplantation in congenic strains of mice. Transplant Proc. 1973 Mar;5(1):733–735. [PubMed] [Google Scholar]
- Damle N. K., Aruffo A. Vascular cell adhesion molecule 1 induces T-cell antigen receptor-dependent activation of CD4+T lymphocytes. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6403–6407. doi: 10.1073/pnas.88.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G. M., Jensen J. C., Alexander H. R., Buresh C. M., Norton J. A. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991 Aug;110(2):192–198. [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Ferran C., Dy M., Sheehan K., Merite S., Schreiber R., Landais P., Grau G., Bluestone J., Bach J. F., Chatenoud L. Inter-mouse strain differences in the in vivo anti-CD3 induced cytokine release. Clin Exp Immunol. 1991 Dec;86(3):537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., Sanderson J. B., Gray D. W., Richardson A., Morris P. J. Variation in expression of endothelial adhesion molecules in pretransplant and transplanted kidneys--correlation with intragraft events. Transplantation. 1993 Jan;55(1):117–123. doi: 10.1097/00007890-199301000-00022. [DOI] [PubMed] [Google Scholar]
- Harning R., Pelletier J., Lubbe K., Takei F., Merluzzi V. J. Reduction in the severity of graft-versus-host disease and increased survival in allogenic mice by treatment with monoclonal antibodies to cell adhesion antigens LFA-1 alpha and MALA-2. Transplantation. 1991 Nov;52(5):842–845. doi: 10.1097/00007890-199111000-00017. [DOI] [PubMed] [Google Scholar]
- Hession C., Moy P., Tizard R., Chisholm P., Williams C., Wysk M., Burkly L., Miyake K., Kincade P., Lobb R. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992 Feb 28;183(1):163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Morris P. J., Austyn J. M. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppink D. M., Bishop D. K., Sedmak D. D., Henry M. L., Ferguson R. M., Streeter P. R., Butcher E. C., Orosz C. G. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation. 1989 Nov;48(5):874–877. doi: 10.1097/00007890-198911000-00032. [DOI] [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. J., Pelletier R. P., Hernandez C. J., Teske D. L., Huang E., Ohye R., Orosz C. G., Ferguson R. M. Alloantigen-dependent endothelial phenotype and lymphokine mRNA expression in rejecting murine cardiac allografts. Transplantation. 1993 Apr;55(4):919–924. doi: 10.1097/00007890-199304000-00042. [DOI] [PubMed] [Google Scholar]
- Norton J., Sloane J. P., al-Saffar N., Haskard D. O. Vessel associated adhesion molecules in normal skin and acute graft-versus-host disease. J Clin Pathol. 1991 Jul;44(7):586–591. doi: 10.1136/jcp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. P., Morgan C. J., Sedmak D. D., Miyake K., Kincade P. W., Ferguson R. M., Orosz C. G. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993 Feb;55(2):315–320. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Prieto J., Takei F., Gendelman R., Christenson B., Biberfeld P., Patarroyo M. MALA-2, mouse homologue of human adhesion molecule ICAM-1 (CD54). Eur J Immunol. 1989 Sep;19(9):1551–1557. doi: 10.1002/eji.1830190906. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Li L. J., Sepp N. T., Caughman S. W., Lawley T. J. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992 Jul 15;149(2):698–705. [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H., Pigott R. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992 Sep;54(3):451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Vonderheide R. H., Springer T. A. Lymphocyte adhesion through very late antigen 4: evidence for a novel binding site in the alternatively spliced domain of vascular cell adhesion molecule 1 and an additional alpha 4 integrin counter-receptor on stimulated endothelium. J Exp Med. 1992 Jun 1;175(6):1433–1442. doi: 10.1084/jem.175.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Newman W., Shimizu Y., Nutman T. B., Tanaka Y., Horgan K. J., Gopal T. V., Ennis E., O'Sullivan D., Grey H. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991 Oct 1;174(4):901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]