Abstract
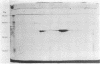
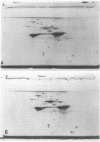
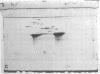
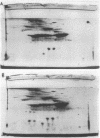
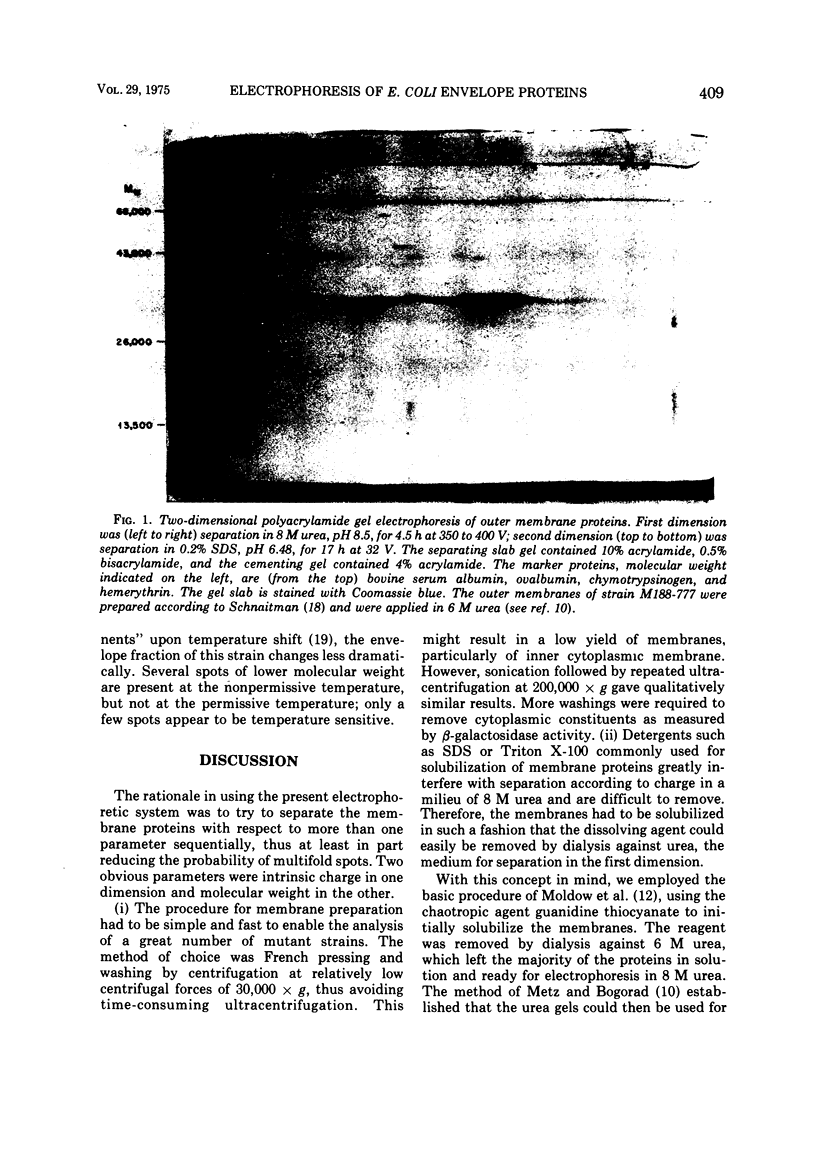
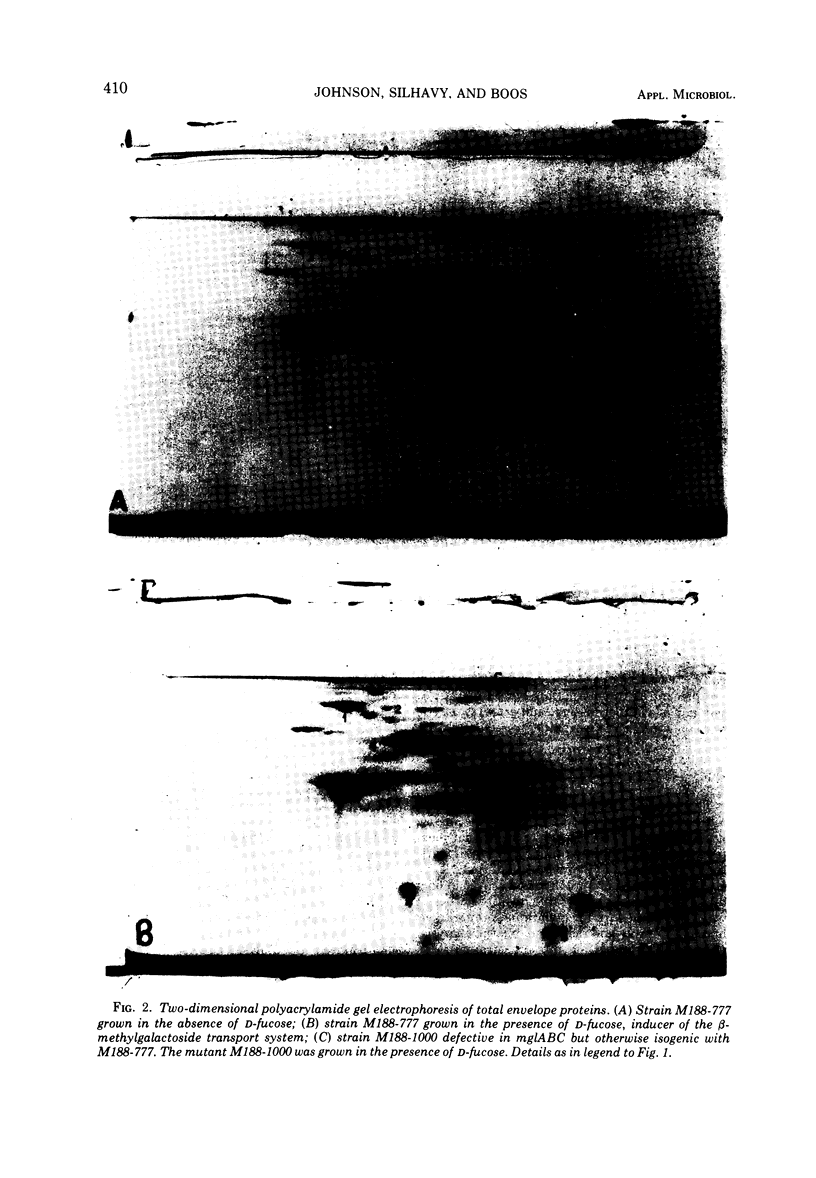
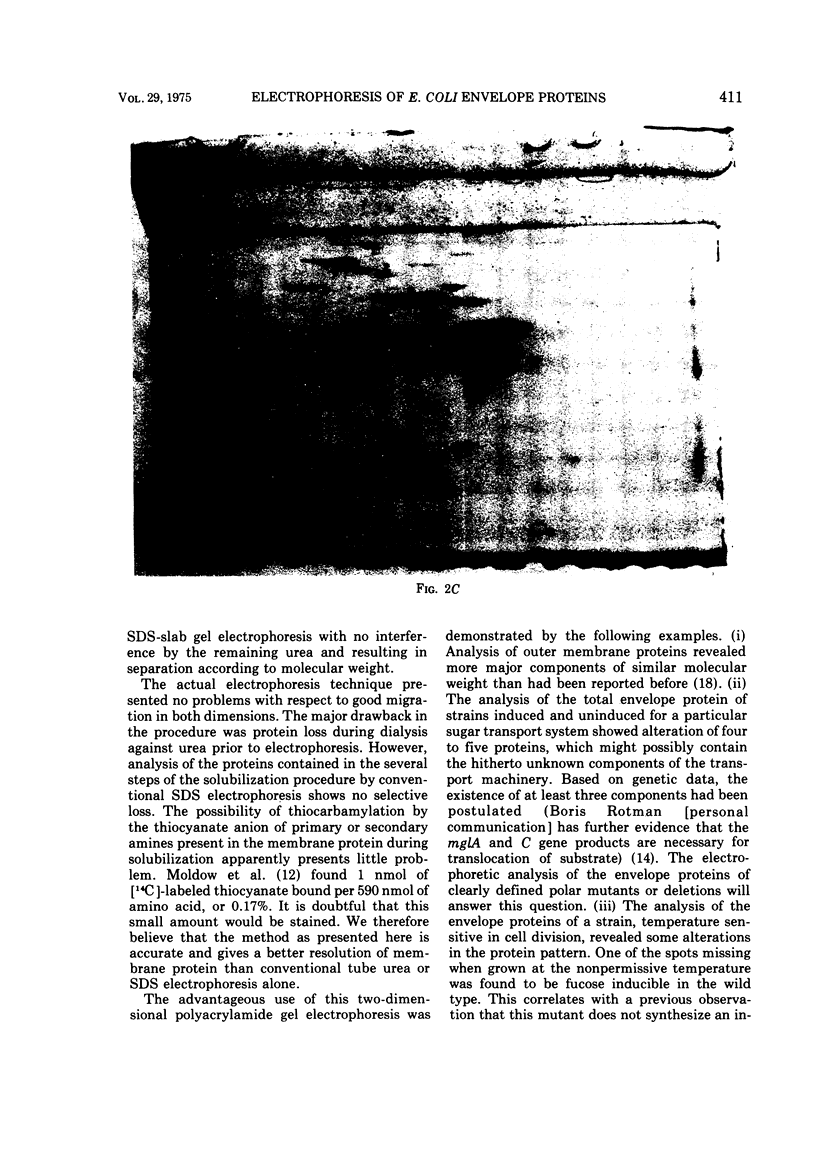
A method of separating envelope proteins by two-dimensional polyacrylamide gel electrophoresis is described. Escherichia coli envelopes (inner and outer membranes) were prepared by French pressing and washed by repeated centrifugation. Membrane proteins were solubilized with guanidine thiocyanate and were dialyzed against urea prior to two-dimensional electrophoretic analysis. The slab gel apparatus and conditions were similar to the technique developed by Metz and Bogorad (1974) for the separation of ribosomal proteins. This separation occurs in 8 M urea for the first dimension and in 0.2% sodium dodecyl sulfate for the second dimension. The technique separates about 70 different membrane proteins in a highly reproducible fashion according to both intrinsic charge and molecular weight. Some examples of alterations in the membrane protein pattern are demonstrated. These alterations are caused by a mutation affecting a sugar transport system and by growth in the presence of D-fucose, inducer of the transport system. A further example of membrane protein changes introduced by growth at the nonpermissive temperature of a temperature-sensitive cell division mutant is shown. Finally, it is demonstrated that the major outer membrane component of Escherichia coli K-12 contains more than four proteins of similar molecular weight.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Two-dimensional polyacrylamide gel electrophoresis: an improved method for ribosomal proteins. Anal Biochem. 1974 Jan;57(1):200–210. doi: 10.1016/0003-2697(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Moldow C., Robertson J., Rothfield L. Purification of bacterial membrane proteins. The use of guanidinium thiocyanate and urea. J Membr Biol. 1972;10(2):137–152. doi: 10.1007/BF01867850. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. H., Boos W. Regulation of the -methylgalactoside transport system and the galatose-binding protein by the cell cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1481–1485. doi: 10.1073/pnas.70.5.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Boos W. Selection procedure for mutants defective in the beta-methylgalactoside transport system of Escherichia coli utilizing the compound 2R-glyceryl-beta-D-galactopyranoside. J Bacteriol. 1974 Oct;120(1):424–432. doi: 10.1128/jb.120.1.424-432.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R. Sensitive separation procedure for Escherichia coli ribosomal proteins and the resolution of high-molecular-weight components. Eur J Biochem. 1974 Jun 15;45(2):541–546. doi: 10.1111/j.1432-1033.1974.tb03579.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wu H. C., Boos W., Kalckar H. M. Role of the galactose transport system in the retention of intracellular galactose in Escherichia coli. J Mol Biol. 1969 Apr 14;41(1):109–120. doi: 10.1016/0022-2836(69)90129-6. [DOI] [PubMed] [Google Scholar]