Abstract
A search was undertaken for bacteria which degrade chondroitin sulfate in nature and to find bacteria with a usefully high rate of chondroitinase (ChSase) productivity. First, 253 ChSase-producing bacteria were obtained from aquatic and land environments in Japan by aerobic and anaerobic screening methods. Identification according to Bergey's Manual of Determinative Bacteriology or Bain and Shewan (1968) permitted assignment of the majority of the isolates to seven genera, Aeromonas, Vibrio, Flavobacterium, Beneckea, Proteus, Micrococcus, and Arthrobacter. Next, ChSase productivities of all the isolates were compared with those of two established ChSase-producing stock strains, Proteus vulgaris NCTC 4636 and Flavobacterium heparinum ATCC 13125. As a result, special attention was given to production by a strain of Aeromonas sp. of large quantities of extracellular ChSase-AC. None of the isolates from the current study displayed significant ChSase-ABC productivity. Finally, ChSase-AC was prepared from the culture fluid of the Aeromonas strain by fractional precipitation with ammonium sulfate, chromatography on phospho-cellulose and diethylaminoethyl-cellulose, and gel filtration on Sephadex G-200. It was concluded that the Aeromonas strain may represent a profitable source of the enzyme ChSase-AC.
Full text
PDF



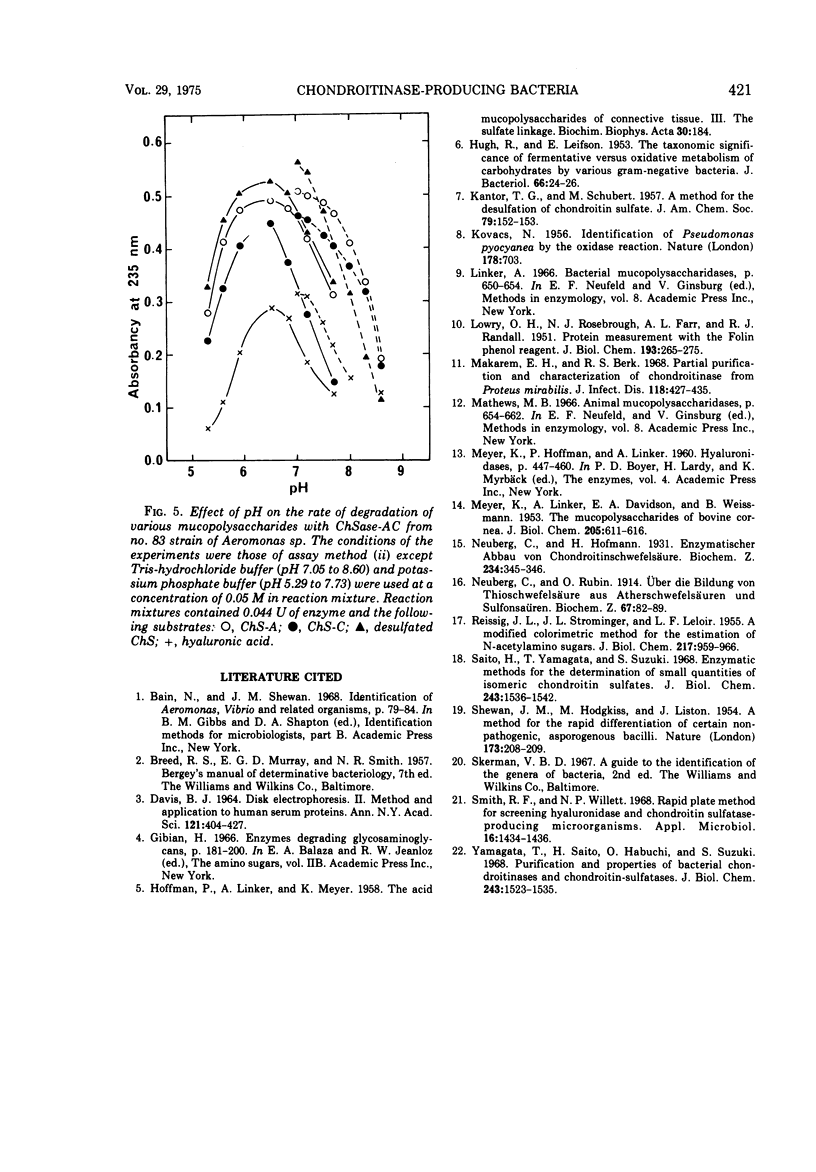
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956 Sep 29;178(4535):703–703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEYER K., LINKER A., DAVIDSON E. A., WEISSMANN B. The mucopolysaccharides of bovine cornea. J Biol Chem. 1953 Dec;205(2):611–616. [PubMed] [Google Scholar]
- Makarem E. H., Berk R. S. Partial purification and characterization of chondroitinase from Proteus mirabilis. J Infect Dis. 1968 Oct;118(4):427–435. doi: 10.1093/infdis/118.4.427. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SHEWAN J. M., HODGKISS W. A method for the rapid differentiation of certain nonpathogenic, asporogenous bacilli. Nature. 1954 Jan 30;173(4396):208–209. doi: 10.1038/173208b0. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]