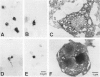
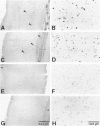
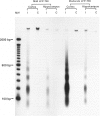
Abstract
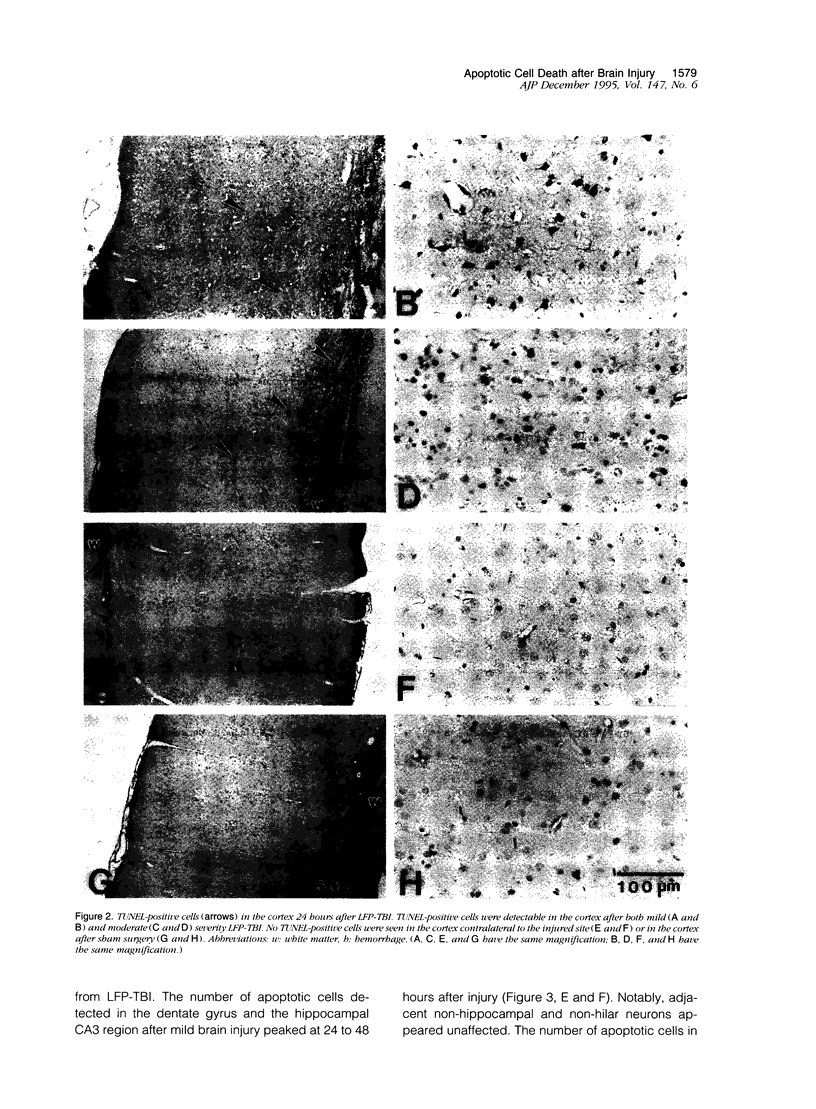
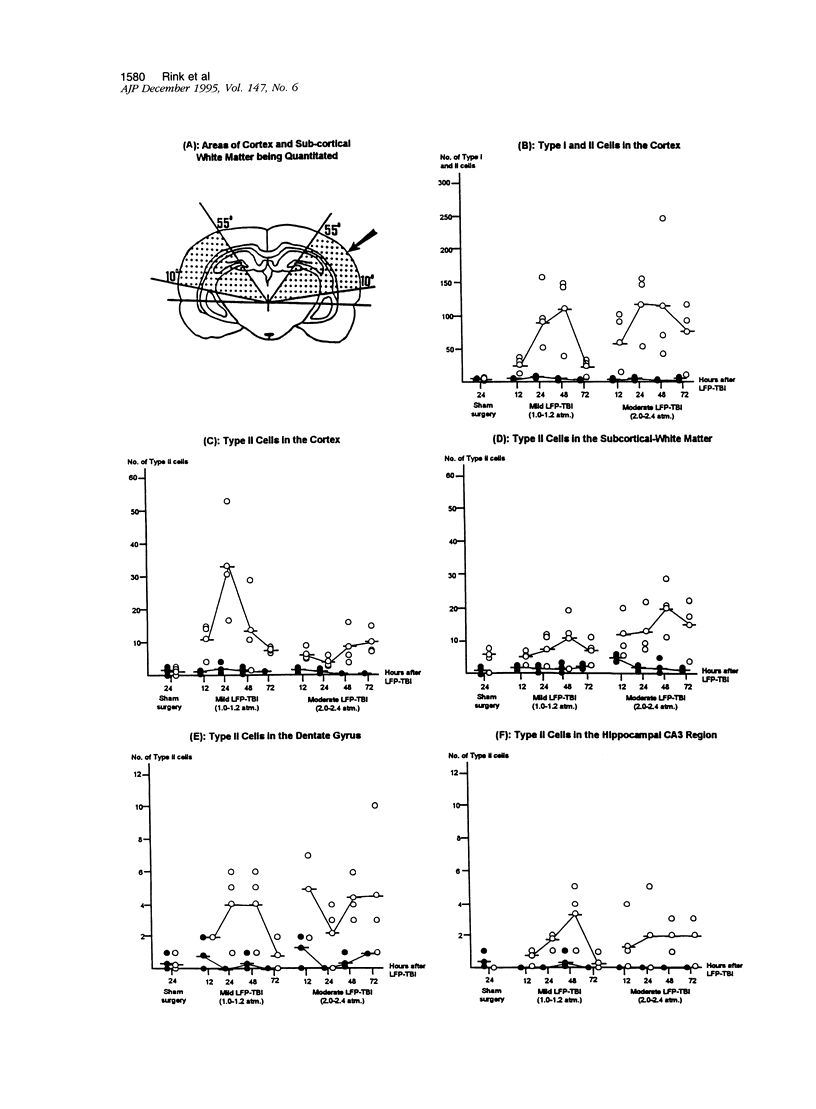
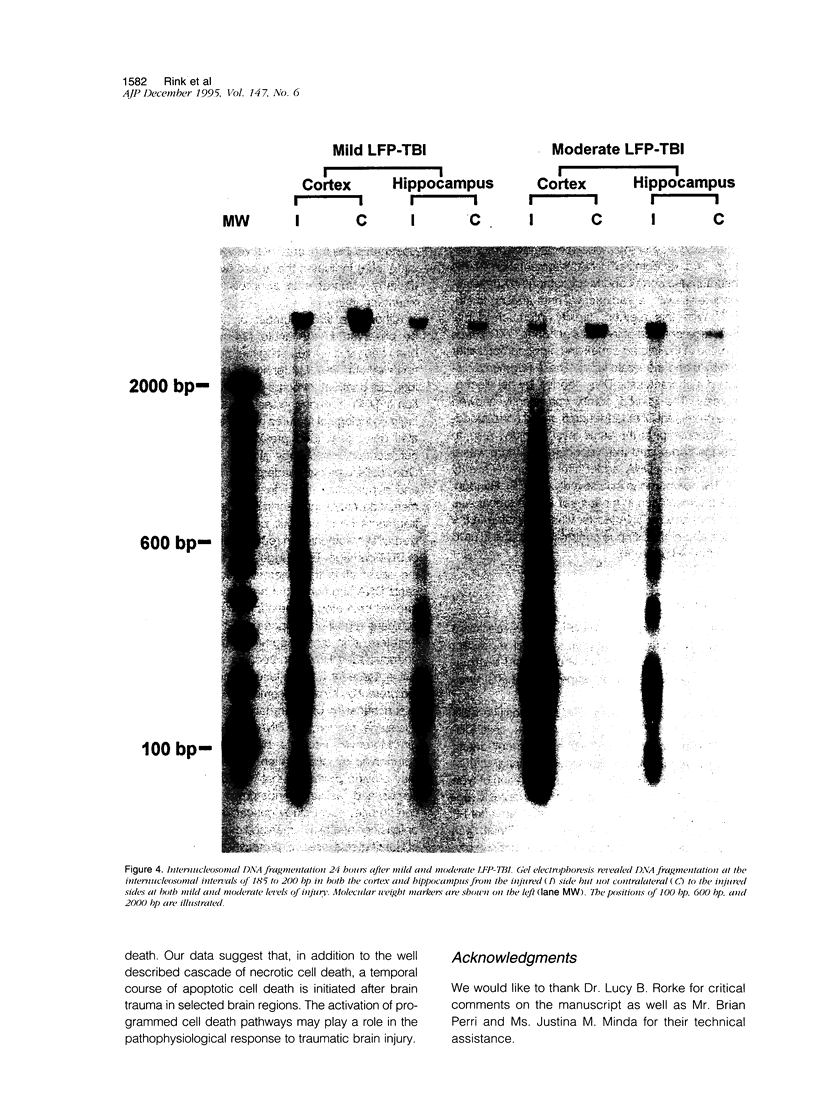
Apoptosis plays an important role in many developmental and pathological processes of the central nervous system. However, the role of apoptosis in traumatic brain injury has not been determined. Using the terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphate nick end labeling (TUNEL) method, we detected many cells with extensive DNA fragmentation in different regions of the brains of rats subjected to experimental traumatic brain injury. Two types of TUNEL-positive cells were demonstrated by light and electron microscopy, including type I cells that displayed morphological features of necrotic cell death and type II cells that displayed morphological features of classic apoptotic cell death. TUNEL-positive cells were detectable for up to 72 hours after the initial injury. Gel electrophoresis of DNA extracted from affected areas of the injured brain containing both type I and II cells revealed only internucleosomal fragmentation at 185-bp intervals, a feature originally described in apoptotic cell death. These data suggest that apoptosis, in addition to necrotic cell death, occurs after traumatic brain injury, and that internucleosomal fragmentation of DNA may be associated with certain types of necrotic cell death.
Full text
PDF


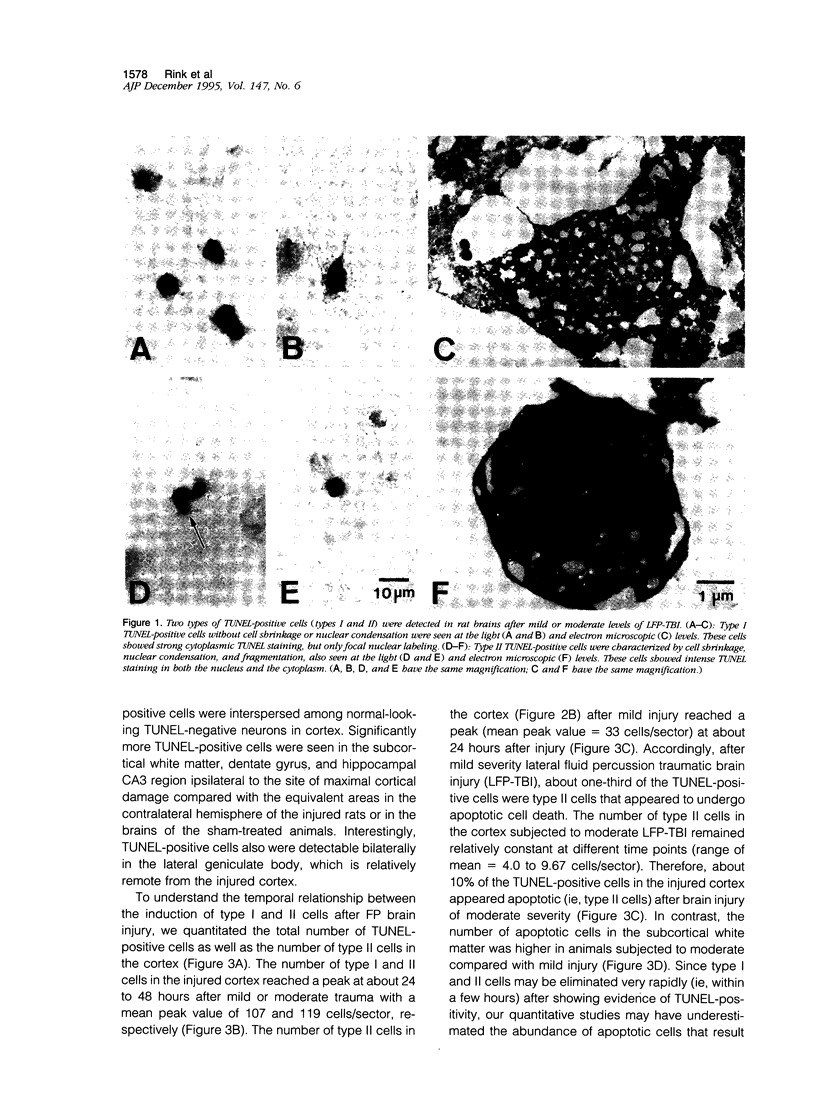


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Doyle D., Ford I., Gennarelli T. A., Graham D. I., McLellan D. R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989 Jul;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Merry D. E., Korsmeyer S. J., Greene L. A. Bcl-2 affects survival but not neuronal differentiation of PC12 cells. J Neurosci. 1993 Oct;13(10):4422–4428. doi: 10.1523/JNEUROSCI.13-10-04422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez S. C., McIntosh T. K., Noble L. J. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989 Mar 20;482(2):271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- Deckwerth T. L., Johnson E. M., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993 Dec;123(5):1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S., Zaks W. J., Freeman R. S., Gruda M., Bravo R., Johnson E. M., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994 Dec;127(6 Pt 1):1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39(2):451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Tortosa A., Macaya A., Sierra A., Moreno D., Munell F., Blanco R., Squier W. Evidence of nuclear DNA fragmentation following hypoxia-ischemia in the infant rat brain, and transient forebrain ischemia in the adult gerbil. Brain Pathol. 1994 Apr;4(2):115–122. doi: 10.1111/j.1750-3639.1994.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kojiro M., Chiu J. F. Demonstration of extensive chromatin cleavage in transplanted Morris hepatoma 7777 tissue: apoptosis or necrosis? Am J Pathol. 1993 Mar;142(3):935–946. [PMC free article] [PubMed] [Google Scholar]
- Fung K. M., Chikaraishi D. M., Suri C., Theuring F., Messing A., Albert D. M., Lee V. M., Trojanowski J. Q. Molecular phenotype of simian virus 40 large T antigen-induced primitive neuroectodermal tumors in four different lines of transgenic mice. Lab Invest. 1994 Jan;70(1):114–124. [PubMed] [Google Scholar]
- Fung K. M., Lee V. M., Trojanowski J. Q. Dynamics of cell proliferation and cell death during the emergence of primitive neuroectodermal tumors of the immature central nervous system in transgenic mice. Am J Pathol. 1995 Jun;146(6):1376–1387. [PMC free article] [PubMed] [Google Scholar]
- Fung K. M., Trojanowski J. Q. Animal models of medulloblastomas and related primitive neuroectodermal tumors. A review. J Neuropathol Exp Neurol. 1995 May;54(3):285–296. doi: 10.1097/00005072-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R. R., Smith D. H., Lowenstein D. H., Saint Marie R., McIntosh T. K. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma. 1993 Winter;10(4):405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]
- Itoh G., Tamura J., Suzuki M., Suzuki Y., Ikeda H., Koike M., Nomura M., Jie T., Ito K. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol. 1995 Jun;146(6):1325–1331. [PMC free article] [PubMed] [Google Scholar]
- Lockshin R. A., Zakeri Z. F. Physiology and protein synthesis in programmed cell death. Early synthesis and DNA degradation. Ann N Y Acad Sci. 1992 Nov 21;663:234–249. doi: 10.1111/j.1749-6632.1992.tb38667.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein D. H., Thomas M. J., Smith D. H., McIntosh T. K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992 Dec;12(12):4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J. C., Dubois-Dauphin M., Staple J. K., Rodriguez I., Frankowski H., Missotten M., Albertini P., Talabot D., Catsicas S., Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994 Oct;13(4):1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- McIntosh T. K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., Faden A. L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28(1):233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C., Hedreen J. C., Price D. L., Koliatsos V. E. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J Neurosci. 1995 May;15(5 Pt 2):3775–3787. doi: 10.1523/JNEUROSCI.15-05-03775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C., Sung C. H., Nathans J., Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock J. T., Erb D. E., Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J Neurotrauma. 1992 Mar;9 (Suppl 1):S189–S200. [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Schiffer D., Cavalla P., Chiò A., Giordana M. T., Marino S., Mauro A., Migheli A. Tumor cell proliferation and apoptosis in medulloblastoma. Acta Neuropathol. 1994;87(4):362–370. doi: 10.1007/BF00313605. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Lowenstein D. H., Gennarelli T. A., McIntosh T. K. Persistent memory dysfunction is associated with bilateral hippocampal damage following experimental brain injury. Neurosci Lett. 1994 Feb 28;168(1-2):151–154. doi: 10.1016/0304-3940(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Tilly J. L., Hsueh A. J. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993 Mar;154(3):519–526. doi: 10.1002/jcp.1041540310. [DOI] [PubMed] [Google Scholar]
- White K., Grether M. E., Abrams J. M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994 Apr 29;264(5159):677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]