Abstract
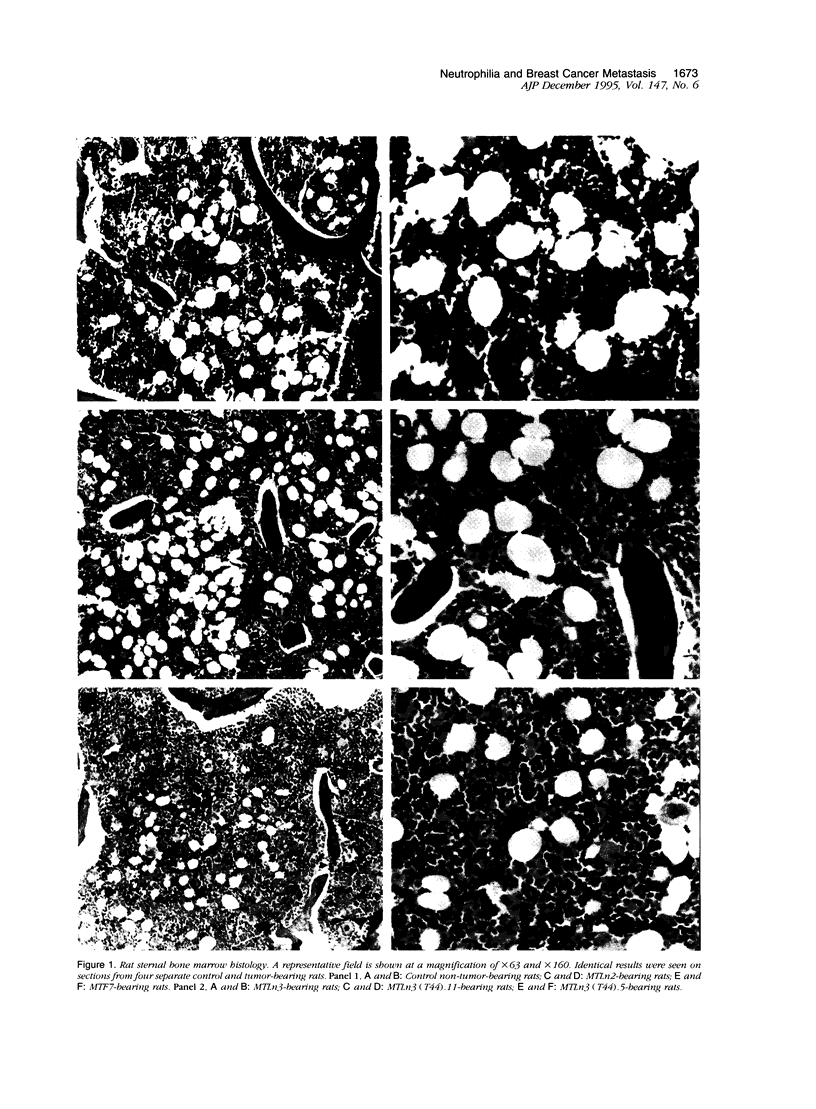
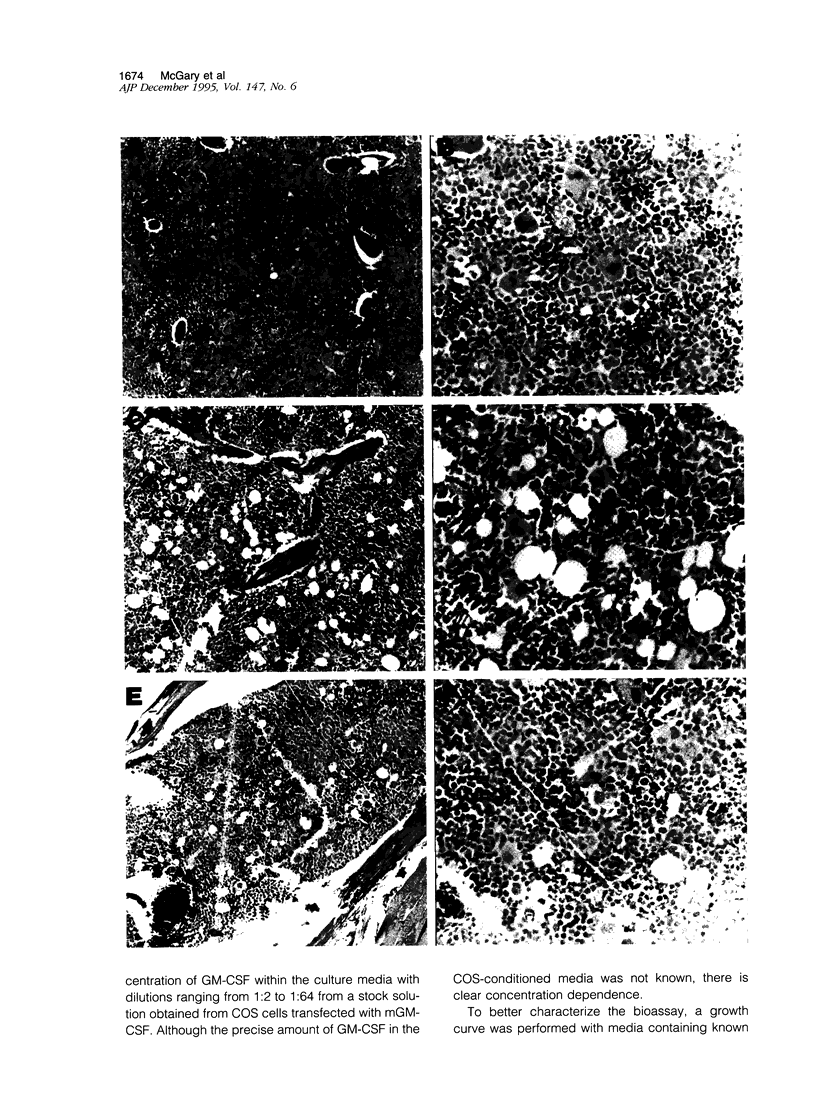
Circulating neutrophil (polymorphonuclear leukocyte levels rise 50-fold in 13762NF tumor-bearing rats in proportion to the tumor's metastatic potential. Purified tumor-elicited neutrophils enhance metastasis of syngeneic tumor cells when co-injected intravenously; however, circulating and phorbol ester-activated polymorphonuclear neutrophils do not. The purpose of this study was to elucidate the source of tumor-elicited neutrophils in metastatic tumor-bearing rats. We examined the bone marrow in rats bearing tumors of poorly, moderately, and highly metastatic cell clones. Marrow from rats with highly metastatic tumors had increased cellularity (100%), myeloid to erythroid ratio (10:1), and megakaryocytes compared with control rats (cellularity, approximately 80%; myeloid to erythroid ratio, 5:1), with marrows from rats with moderately metastatic tumors having intermediate values. This suggested production of a colony-stimulating factor by the metastatic cells. To confirm this, bone marrow colony formation from control and tumor-bearing rats was compared. Colony number increased in proportion to the metastatic potential of the tumor. Conditioned medium from metastatic cells supported growth of the granulocyte-macrophage colony-stimulating factor/interleukin-3-dependent 32Dcl3 cell line, but media from nonmetastatic or moderately metastatic cells did not. Antibodies to murine granulocyte-macrophage colony-stimulating factor neutralized 32Dcl3 growth in tumor cell conditioned medium. These results suggest production of a granulocyte-macrophage colony-stimulating factor or interleukin-3-like activity by highly metastatic 13762NF clones and implicate a possible role for colony-stimulating factors in regulating the metastatic potential of mammary adenocarcinoma cell clones.
Full text
PDF




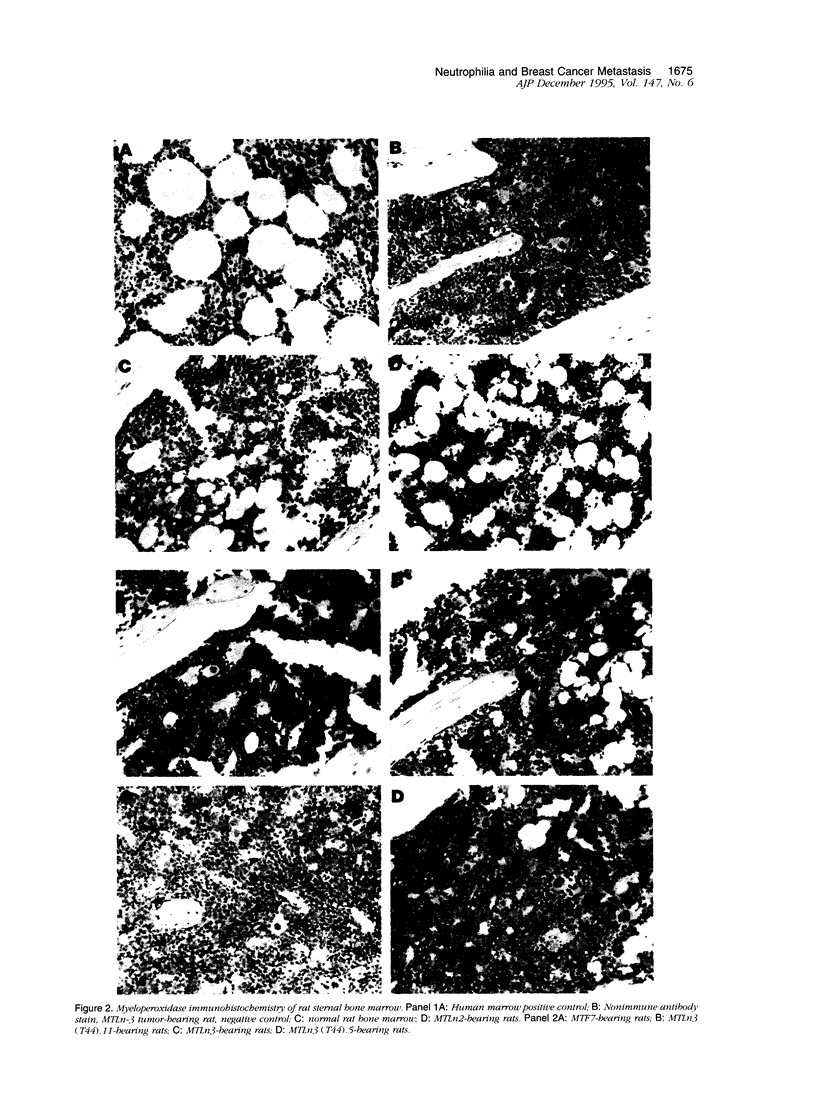
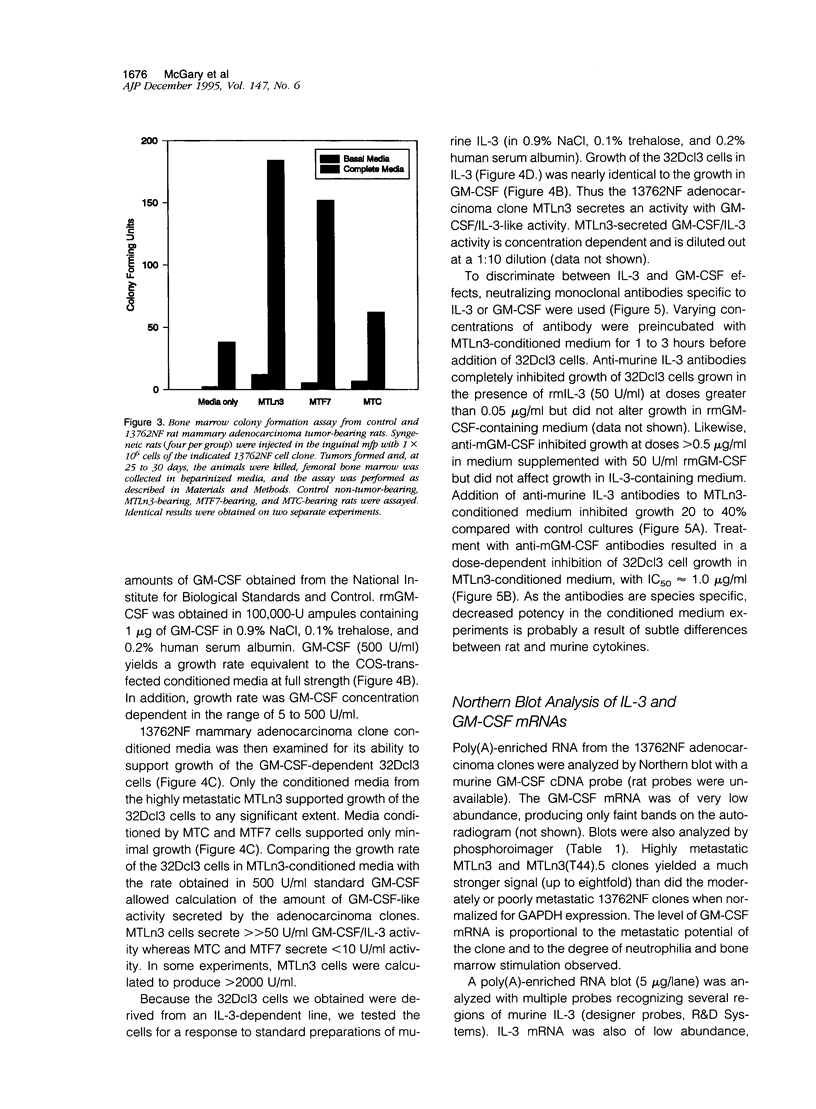
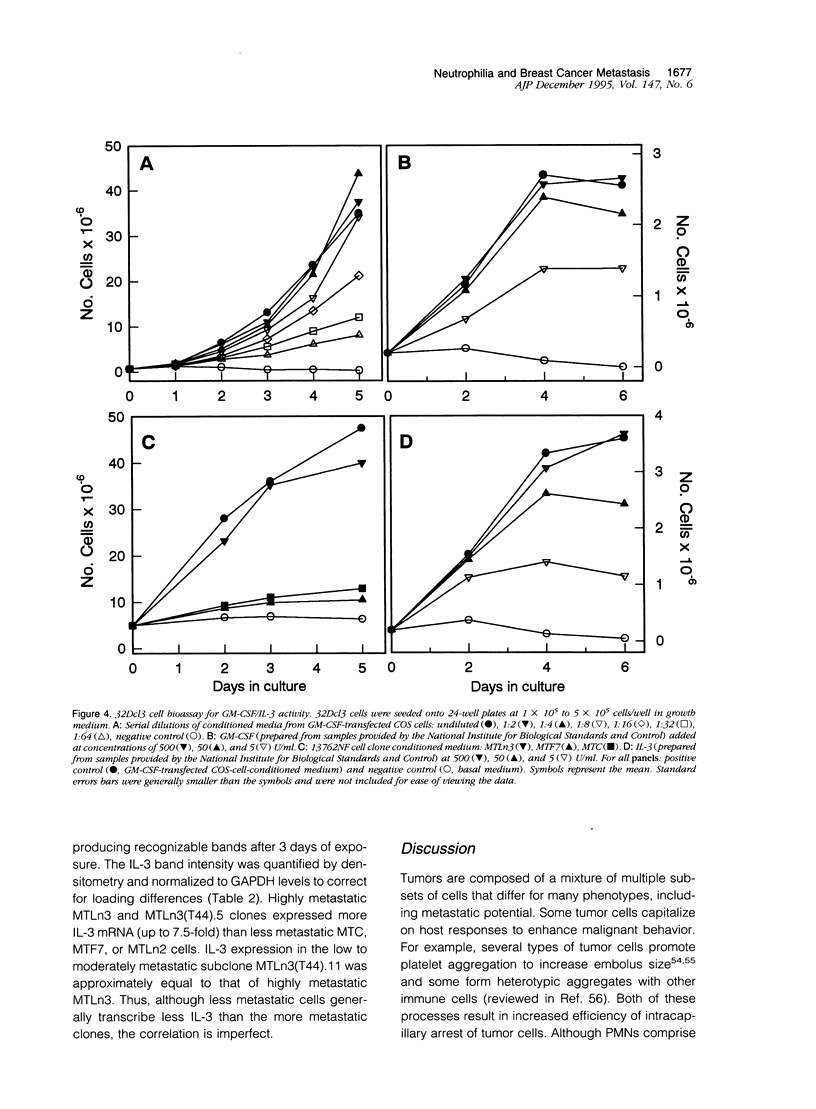
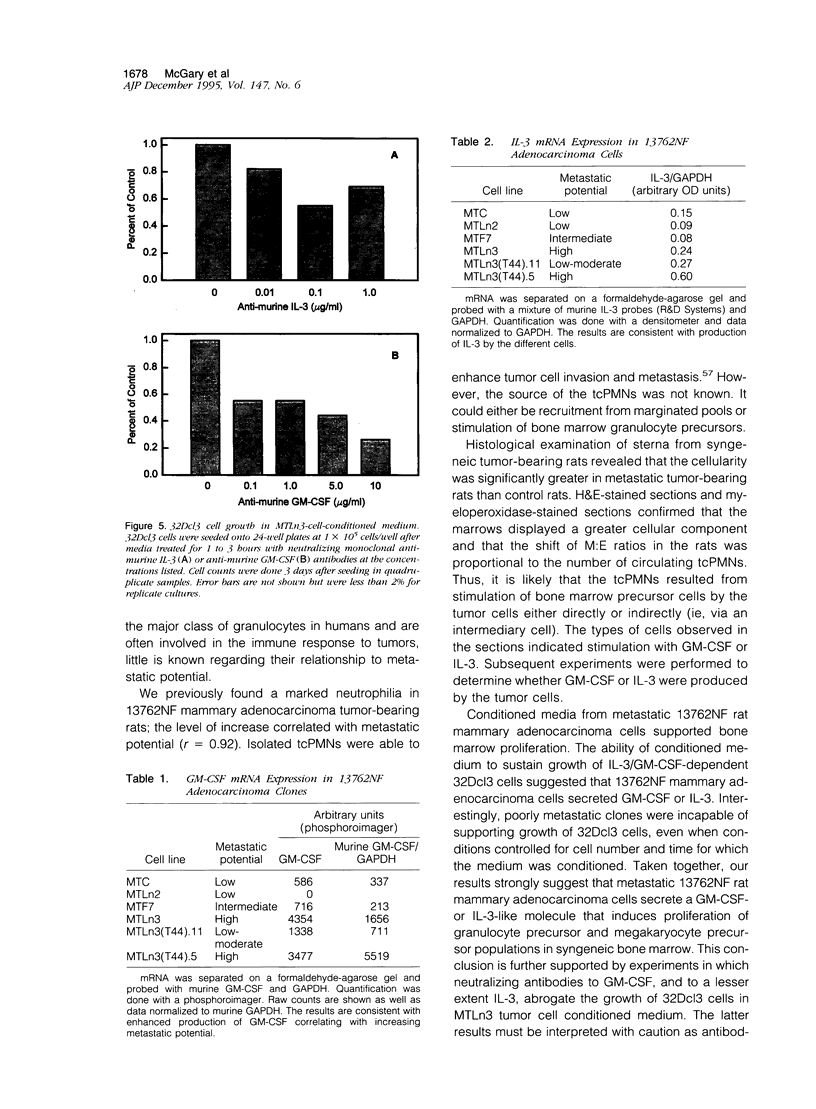



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. G., Bhuyan B. K. Effects of 7-R-O-methylnogarol (menogaril) on L1210 cell progression in vitro and in vivo. Cancer Res. 1986 Dec;46(12 Pt 1):6125–6130. [PubMed] [Google Scholar]
- Aeed P. A., Nakajima M., Welch D. R. The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer. 1988 Nov 15;42(5):748–759. doi: 10.1002/ijc.2910420521. [DOI] [PubMed] [Google Scholar]
- Asano S., Urabe A., Okabe T., Sato N., Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977 May;49(5):845–852. [PubMed] [Google Scholar]
- Barker E., Reisfeld R. A. A mechanism for neutrophil-mediated lysis of human neuroblastoma cells. Cancer Res. 1993 Jan 15;53(2):362–367. [PubMed] [Google Scholar]
- Berkow R. L. Granulocyte-macrophage colony-stimulating factor induces a staurosporine inhibitable tyrosine phosphorylation of unique neutrophil proteins. Blood. 1992 May 1;79(9):2446–2454. [PubMed] [Google Scholar]
- Cameron D. J. Role of hydrocortisone, chloroquine and prednisolone in neutrophil mediated cytotoxicity. Jpn J Exp Med. 1986 Oct;56(5):207–212. [PubMed] [Google Scholar]
- Conti P., Reale M., Barbacane R. C., Panara M. R., Bongrazio M., Fiore S., Mier J. W., Dempsey R. A. Granulocyte-macrophage colony stimulating factor potentiates human polymorphonuclear leukocyte aggregation responses to formyl-methionyl-leucyl-phenylalanine. Immunol Lett. 1992 Mar;32(1):71–79. doi: 10.1016/0165-2478(92)90202-y. [DOI] [PubMed] [Google Scholar]
- Corey S. J., Burkhardt A. L., Bolen J. B., Geahlen R. L., Tkatch L. S., Tweardy D. J. Granulocyte colony-stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4683–4687. doi: 10.1073/pnas.91.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman J. D., Hatfield J., Schaldenbrand M., Sloane B. F., Honn K. V. Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Lab Invest. 1985 Oct;53(4):470–478. [PubMed] [Google Scholar]
- Demetri G. D., Antman K. H. Granulocyte-macrophage colony-stimulating factor (GM-CSF): preclinical and clinical investigations. Semin Oncol. 1992 Aug;19(4):362–385. [PubMed] [Google Scholar]
- Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994 Apr 29;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Estrada J., Nicolson G. L. Tumor-cell-platelet aggregation does not correlate with metastatic potential of rat 13762NF mammary adenocarcinoma tumor cell clones. Int J Cancer. 1984 Jul 15;34(1):101–105. doi: 10.1002/ijc.2910340118. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Fisher B., Saffer E. A. Tumor cells cytotoxicity by granulocytes from peripheral blood of tumor-bearing mice. J Natl Cancer Inst. 1978 Mar;60(3):687–691. doi: 10.1093/jnci/60.3.687. [DOI] [PubMed] [Google Scholar]
- Fu Y. X., Watson G. A., Kasahara M., Lopez D. M. The role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. I. Induction of regulatory macrophages in normal mice by the in vivo administration of rGM-CSF. J Immunol. 1991 Jan 15;146(2):783–789. [PubMed] [Google Scholar]
- Garofalo A., Chirivi R. G., Scanziani E., Mayo J. G., Vecchi A., Giavazzi R. Comparative study on the metastatic behavior of human tumors in nude, beige/nude/xid and severe combined immunodeficient mice. Invasion Metastasis. 1993;13(2):82–91. [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983 Nov;48(5):665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E., Wiltrout R. H., Brunda M. J., Holden H. T., Herberman R. B. Augmentation of metastasis formation by thioglycollate-elicited macrophages. Int J Cancer. 1982 May 15;29(5):575–581. doi: 10.1002/ijc.2910290514. [DOI] [PubMed] [Google Scholar]
- HUGHES W. F., HIGLEY C. S. Marked leukocytosis resulting from carcinomatosis. Ann Intern Med. 1952 Nov;37(5):1085–1088. doi: 10.7326/0003-4819-37-5-1085. [DOI] [PubMed] [Google Scholar]
- Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta. 1985;780(3):213–226. doi: 10.1016/0304-419x(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G., Chen Y. Q. Platelets and cancer metastasis: more than an epiphenomenon. Semin Thromb Hemost. 1992;18(4):392–415. doi: 10.1055/s-2007-1002578. [DOI] [PubMed] [Google Scholar]
- Hoover H. C., Jr, Ketcham A. S. Techniques for inhibiting tumor metastases. Cancer. 1975 Jan;35(1):5–14. doi: 10.1002/1097-0142(197501)35:1<5::aid-cncr2820350103>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ikenami M., Mizuno D., Yamazaki M. Drug-dependent cellular cytotoxicity mediated by polymorphonuclear leukocytes. Jpn J Cancer Res. 1985 Jul;76(7):637–643. [PubMed] [Google Scholar]
- Inoue T., Sendo F. In vitro induction of cytotoxic polymorphonuclear leukocytes by supernatant from a concanavalin A-stimulated spleen cell culture. J Immunol. 1983 Nov;131(5):2508–2514. [PubMed] [Google Scholar]
- Key M. E. Macrophages in cancer metastases and their relevance to metastatic growth. Cancer Metastasis Rev. 1983;2(1):75–88. doi: 10.1007/BF00046906. [DOI] [PubMed] [Google Scholar]
- Klotz K. N., Jesaitis A. J. Neutrophil chemoattractant receptors and the membrane skeleton. Bioessays. 1994 Mar;16(3):193–198. doi: 10.1002/bies.950160310. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Hollister G. H., DiPersio J. D., Wahl S., Liotta L. A., Schiffmann E. Granulocyte-macrophage colony-stimulating factor induces human melanoma-cell migration. Int J Cancer. 1993 Apr 1;53(6):968–972. doi: 10.1002/ijc.2910530618. [DOI] [PubMed] [Google Scholar]
- Kondo M., Kato H., Yoshikawa T., Sugino S. Treatment of cancer ascites by intraperitoneal administration of a streptococcal preparation OK-432 with fresh human complement--role of complement-derived chemotactic factor to neutrophils. Int J Immunopharmacol. 1986;8(7):715–721. doi: 10.1016/0192-0561(86)90007-x. [DOI] [PubMed] [Google Scholar]
- Kripke M. L. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994 Dec 1;54(23):6102–6105. [PubMed] [Google Scholar]
- Lee M. Y., Baylink D. J. Hypercalcemia, excessive bone resorption, and neutrophilia in mice bearing a mammary carcinoma. Proc Soc Exp Biol Med. 1983 Apr;172(4):424–429. doi: 10.3181/00379727-172-41582. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Liu C. C., Lottsfeldt J. L., Judkins S. A., Howard G. A. Production of granulocyte-stimulating and bone cell-modulating activities from a neutrophilia hypercalcemia-inducing murine mammary cancer cell line. Cancer Res. 1987 Aug 1;47(15):4059–4065. [PubMed] [Google Scholar]
- Lee M. Y., Lottsfeldt J. L. Augmentation of neutrophilic granulocyte progenitors in the bone marrow of mice with tumor-induced neutrophilia: cytochemical study of in vitro colonies. Blood. 1984 Aug;64(2):499–506. [PubMed] [Google Scholar]
- Mano H., Nishida J., Usuki K., Maru Y., Kobayashi Y., Hirai H., Okabe T., Urabe A., Takaku F. Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in human solid tumors. Jpn J Cancer Res. 1987 Oct;78(10):1041–1043. [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994 Jul;71(1):5–16. [PubMed] [Google Scholar]
- Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991 Oct 25;254(5031):529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Hemopoietic regulators. Trends Biochem Sci. 1992 Aug;17(8):286–289. doi: 10.1016/0968-0004(92)90436-d. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Welch D., Kawaguchi T., Nicolson G. L. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982 Mar;68(3):507–517. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988 Nov 15;948(2):175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Growth mechanisms and cancer progression. Hosp Pract (Off Ed) 1993 Feb 15;28(2):43–53. doi: 10.1080/21548331.1993.11442751. [DOI] [PubMed] [Google Scholar]
- Nitta T., Sato K., Allegretta M., Brocke S., Lim M., Mitchell D. J., Steinman L. Expression of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor genes in human astrocytoma cell lines and in glioma specimens. Brain Res. 1992 Jan 31;571(1):19–25. doi: 10.1016/0006-8993(92)90505-4. [DOI] [PubMed] [Google Scholar]
- Pickaver A. H., Ratcliffe N. A., Williams A. E., Smith H. Cytotoxic effects of peritoneal neutrophils on a syngeneic rat tumour. Nat New Biol. 1972 Feb 9;235(58):186–187. doi: 10.1038/newbio235186a0. [DOI] [PubMed] [Google Scholar]
- Price J. E. Host-tumor interactions in the progression of breast cancer metastasis. In Vivo. 1994 Jan-Feb;8(1):145–154. [PubMed] [Google Scholar]
- Quesenberry P. J., Lowry P. A. The colony-stimulating factors. An overview. Cancer. 1992 Aug 15;70(4 Suppl):909–912. [PubMed] [Google Scholar]
- Raines M. A., Liu L., Quan S. G., Joe V., DiPersio J. F., Golde D. W. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8203–8207. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. L., Golde D. W., Quan S., Nimer S. D. Production of granulocyte-macrophage colony-stimulating factor in two patients with lung cancer, leukocytosis, and eosinophilia. Cancer. 1992 Mar 15;69(6):1342–1346. doi: 10.1002/1097-0142(19920315)69:6<1342::aid-cncr2820690607>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Smith J. A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994 Dec;56(6):672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Starkey J. R., Liggitt H. D., Jones W., Hosick H. L. Influence of migratory blood cells on the attachment of tumor cells to vascular endothelium. Int J Cancer. 1984 Oct 15;34(4):535–543. doi: 10.1002/ijc.2910340417. [DOI] [PubMed] [Google Scholar]
- Steward W. P. Granulocyte and granulocyte-macrophage colony-stimulating factors. Lancet. 1993 Jul 17;342(8864):153–157. doi: 10.1016/0140-6736(93)91350-u. [DOI] [PubMed] [Google Scholar]
- Suda T., Miura Y., Mizoguchi H., Kubota K., Takaku F. A case of lung cancer associated with granulocytosis and production of colony-stimulating activity by the tumour. Br J Cancer. 1980 Jun;41(6):980–984. doi: 10.1038/bjc.1980.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Hatakeyama K., Tsuchiya Y., Rikiishi H., Kumagai K. A correlation between GM-CSF gene expression and metastases in murine tumors. Int J Cancer. 1991 Feb 1;47(3):413–420. doi: 10.1002/ijc.2910470318. [DOI] [PubMed] [Google Scholar]
- Tarin D., Matsumura Y. Recent advances in the study of tumour invasion and metastasis. J Clin Pathol. 1994 May;47(5):385–390. doi: 10.1136/jcp.47.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasovic S. P., Armour E. P., North S. M., Welch D. R. Rat mammary adenocarcinoma heat-stress proteins in vivo. Int J Hyperthermia. 1987 Sep-Oct;3(5):467–473. doi: 10.3109/02656738709140417. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Evans D. P., Tomasovic S. P., Milas L., Nicolson G. L. Multiple phenotypic divergence of mammary adenocarcinoma cell clones. II. Sensitivity to radiation, hyperthermia and FUdR. Clin Exp Metastasis. 1984 Oct-Dec;2(4):357–371. doi: 10.1007/BF00135173. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Krizman D. B., Nicolson G. L. Multiple phenotypic divergence of mammary adenocarcinoma cell clones. I. In vitro and in vivo properties. Clin Exp Metastasis. 1984 Oct-Dec;2(4):333–355. doi: 10.1007/BF00135172. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Neri A., Nicolson G. L. Comparison of 'spontaneous' and 'experimental' metastasis using rat 13762 mammary adenocarcinoma metastatic cell clones. Invasion Metastasis. 1983;3(2):65–80. [PubMed] [Google Scholar]
- Welch D. R., Schissel D. J., Howrey R. P., Aeed P. A. Tumor-elicited polymorphonuclear cells, in contrast to "normal" circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5859–5863. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. F. The cytolytic and regulatory role of natural killer cells in experimental neoplasia. Biochim Biophys Acta. 1986 Aug 5;865(1):43–57. doi: 10.1016/0304-419x(86)90012-0. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Wu M., Cini J. K., Yunis A. A. Purification of a colony-stimulating factor from cultured pancreatic carcinoma cells. J Biol Chem. 1979 Jul 25;254(14):6226–6228. [PubMed] [Google Scholar]
- Yong K. L., Linch D. C. Granulocyte-macrophage-colony-stimulating factor differentially regulates neutrophil migration across IL-1-activated and nonactivated human endothelium. J Immunol. 1993 Mar 15;150(6):2449–2456. [PubMed] [Google Scholar]
- Young M. R., Halpin J., Hussain R., Lozano Y., Djordjevic A., Devata S., Matthews J. P., Wright M. A. Inhibition of tumor production of granulocyte-macrophage colony-stimulating factor by 1 alpha, 25-dihydroxyvitamin D3 reduces tumor motility and metastasis. Invasion Metastasis. 1993;13(4):169–177. [PubMed] [Google Scholar]