Abstract
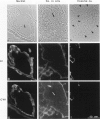
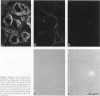
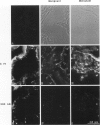
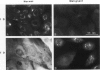
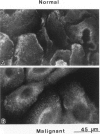
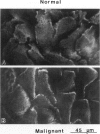
Hemidesmosomes are multiprotein structures that attach basal cells of stratified epithelia to basement membranes. Although normal human breast epithelia are not stratified, we observed expression of electron-dense hemidesmosomes and hemidesmosome protein components by breast epithelial and myoepithelial cells at the basal lamina in vivo. Primary cultured normal human breast epithelial cells also contained hemidesmosomes and component proteins, and could be used as a model for hemidesmosome assembly and regulation. In these cultured cells, hemidesmosome proteins were expressed and localized basally in an unvaried temporal pattern, and electron-dense hemidesmosomes were not seen until the final protein was localized to the cell base. In addition, rate of localization was influenced by confluence, doubling time, and extracellular matrix. Invasive breast cancer cells did not express hemidesmosomes or most of the component proteins in vivo. In carcinoma in situ, cells away from the basement membrane lacked hemidesmosomes and hemidesmosome proteins, and cells at the basement membrane exhibited abnormalities of hemidesmosome protein expression. Primary human malignant breast cells in culture exhibited a mix of hemidesmosome phenotypes. These data suggest that hemidesmosomes may be important subcellular structures in determining the cytoarchitecture of the breast epithelium. Further, their downregulation may influence cytoarchitecture remodeling closely linked with cell cycle, motility, and extracellular matrix interactions; and their loss in carcinoma may be associated with loss of normal cytoarchitecture.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimoto Y., Obinata A., Endo H., Hirano H. Reconstruction of basement membrane in recombinants of epidermis and dermis of chick embryonic skin in vitro: an electron microscopic study. Anat Rec. 1991 Nov;231(3):375–382. doi: 10.1002/ar.1092310311. [DOI] [PubMed] [Google Scholar]
- Albelda S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993 Jan;68(1):4–17. [PubMed] [Google Scholar]
- Behrens J. Cadherins as determinants of tissue morphology and suppressors of invasion. Acta Anat (Basel) 1994;149(3):165–169. doi: 10.1159/000147572. [DOI] [PubMed] [Google Scholar]
- Bergstraesser L. M., Weitzman S. A. Culture of normal and malignant primary human mammary epithelial cells in a physiological manner simulates in vivo growth patterns and allows discrimination of cell type. Cancer Res. 1993 Jun 1;53(11):2644–2654. [PubMed] [Google Scholar]
- Briggaman R. A., Dalldorf F. G., Wheeler C. E., Jr Formation and origin of basal lamina and anchoring fibrils in adult human skin. J Cell Biol. 1971 Nov;51(21):384–395. doi: 10.1083/jcb.51.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggaman R. A., Wheeler C. E., Jr The epidermal-dermal junction. J Invest Dermatol. 1975 Jul;65(1):71–84. doi: 10.1111/1523-1747.ep12598050. [DOI] [PubMed] [Google Scholar]
- Brodt P., Fallavollita L., Sawka R. J., Shibata P., Nip J., Kim U., Shibata H. Tumor cell adhesion to frozen lymph node sections--a correlate of lymphatic metastasis in breast carcinoma models of human and rat origin. Breast Cancer Res Treat. 1990 Dec;17(2):109–120. doi: 10.1007/BF01806291. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. J., Eady R. A. Blistering in keratinocyte cultures: a regular phenomenon associated with differentiation. Eur J Cell Biol. 1986 Jan;39(2):352–359. [PubMed] [Google Scholar]
- Clermont Y., Xia L., Turner J. D., Hermo L. Striated anchoring fibrils-anchoring plaque complexes and their relation to hemidesmosomes of myoepithelial and secretory cells in mammary glands of lactating rats. Anat Rec. 1993 Nov;237(3):318–325. doi: 10.1002/ar.1092370304. [DOI] [PubMed] [Google Scholar]
- Croft C. B., Tarin D. Ultrastructural studies of wound healing in mouse skin. I. Epithelial behaviour. J Anat. 1970 Jan;106(Pt 1):63–77. [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne A. J., Richman P. I., Horton M. A., Mcaulay A. E., Jordan S. Co-ordinate expression of the alpha-6 integrin laminin receptor sub-unit and laminin in breast cancer. J Pathol. 1991 Nov;165(3):213–220. doi: 10.1002/path.1711650304. [DOI] [PubMed] [Google Scholar]
- Eady R. A. The basement membrane. Interface between the epithelium and the dermis: structural features. Arch Dermatol. 1988 May;124(5):709–712. doi: 10.1001/archderm.124.5.709. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr Molecular genetics of epidermolysis bullosa. Science. 1992 May 8;256(5058):799–804. doi: 10.1126/science.1375393. [DOI] [PubMed] [Google Scholar]
- Falcioni R., Sacchi A., Resau J., Kennel S. J. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 1988 Feb 15;48(4):816–821. [PubMed] [Google Scholar]
- Fawcett J., Harris A. L. Cell adhesion molecules and cancer. Curr Opin Oncol. 1992 Feb;4(1):142–148. doi: 10.1097/00001622-199202000-00019. [DOI] [PubMed] [Google Scholar]
- Fine J. D., Smith L. T., Holbrook K. A., Katz S. I. The appearance of four basement membrane zone antigens in developing human fetal skin. J Invest Dermatol. 1984 Jul;83(1):66–69. doi: 10.1111/1523-1747.ep12261707. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S. J., Tisdale A. S. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol. 1988 Apr;126(2):253–262. doi: 10.1016/0012-1606(88)90136-4. [DOI] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S., Tisdale A., Elwell J., Stepp M. A. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp Cell Res. 1993 Jul;207(1):86–98. doi: 10.1006/excr.1993.1166. [DOI] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S., Tisdale A., Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):425–434. [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Hieda Y., Nishizawa Y., Uematsu J., Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992 Mar;116(6):1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintner H., Fritsch P. O., Foidart J. M., Stingl G., Schuler G., Katz S. I. Expression of basement membrane zone antigens at the dermo-epibolic junction in organ cultures of human skin. J Invest Dermatol. 1980 Apr;74(4):200–204. doi: 10.1111/1523-1747.ep12541715. [DOI] [PubMed] [Google Scholar]
- Hopkinson S. B., Riddelle K. S., Jones J. C. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992 Sep;99(3):264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Asmuth J., Baker S. E., Langhofer M., Roth S. I., Hopkinson S. B. Hemidesmosomes: extracellular matrix/intermediate filament connectors. Exp Cell Res. 1994 Jul;213(1):1–11. doi: 10.1006/excr.1994.1166. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Kurpakus M. A., Cooper H. M., Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991 Jun;2(6):427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C., Steinman H. K., Goldsmith B. A. Hemidesmosomes, collagen VII, and intermediate filaments in basal cell carcinoma. J Invest Dermatol. 1989 Nov;93(5):662–671. doi: 10.1111/1523-1747.ep12319833. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Varner J. A. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993 Oct;5(5):812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Khodadoust A. A., Silverstein A. M., Kenyon D. R., Dowling J. E. Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol. 1968 Mar;65(3):339–348. doi: 10.1016/0002-9394(68)93082-1. [DOI] [PubMed] [Google Scholar]
- Klatte D. H., Kurpakus M. A., Grelling K. A., Jones J. C. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Kurpakus M. A., Jones J. C. A novel hemidesmosomal plaque component: tissue distribution and incorporation into assembling hemidesmosomes in an in vitro model. Exp Cell Res. 1991 May;194(1):139–146. doi: 10.1016/0014-4827(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Kurpakus M. A., Quaranta V., Jones J. C. Surface relocation of alpha 6 beta 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991 Dec;115(6):1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus M. A., Stock E. L., Jones J. C. Analysis of wound healing in an in vitro model: early appearance of laminin and a 125 x 10(3) Mr polypeptide during adhesion complex formation. J Cell Sci. 1990 Aug;96(Pt 4):651–660. doi: 10.1242/jcs.96.4.651. [DOI] [PubMed] [Google Scholar]
- Langhofer M., Hopkinson S. B., Jones J. C. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993 Jul;105(Pt 3):753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Legan P. K., Collins J. E., Garrod D. R. The molecular biology of desmosomes and hemidesmosomes: "what's in a name"? Bioessays. 1992 Jun;14(6):385–393. doi: 10.1002/bies.950140608. [DOI] [PubMed] [Google Scholar]
- Mann P. R., Constable H. Induction of basal lamina formation in epidermal cell cultures in vitro. Br J Dermatol. 1977 Apr;96(4):421–426. doi: 10.1111/j.1365-2133.1977.tb07138.x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructural comparison of the interface between epithelium and stroma in basal cell carcinoma and control human skin. Lab Invest. 1976 Aug;35(2):132–142. [PubMed] [Google Scholar]
- Mutasim D. F., Takahashi Y., Labib R. S., Anhalt G. J., Patel H. P., Diaz L. A. A pool of bullous pemphigoid antigen(s) is intracellular and associated with the basal cell cytoskeleton-hemidesmosome complex. J Invest Dermatol. 1985 Jan;84(1):47–53. doi: 10.1111/1523-1747.ep12274684. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Botti C., Mottolese M., Bigotti A., Segatto O. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer. 1992 Aug;66(2):318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod E. J., Rudland P. S. Regeneration of mammary glands in vivo from isolated mammary ducts. J Embryol Exp Morphol. 1986 Jul;96:229–243. [PubMed] [Google Scholar]
- Owaribe K., Kartenbeck J., Stumpp S., Magin T. M., Krieg T., Diaz L. A., Franke W. W. The hemidesmosomal plaque. I. Characterization of a major constituent protein as a differentiation marker for certain forms of epithelia. Differentiation. 1990 Dec;45(3):207–220. doi: 10.1111/j.1432-0436.1990.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Owaribe K., Nishizawa Y., Franke W. W. Isolation and characterization of hemidesmosomes from bovine corneal epithelial cells. Exp Cell Res. 1991 Feb;192(2):622–630. doi: 10.1016/0014-4827(91)90084-8. [DOI] [PubMed] [Google Scholar]
- Ozzello L., Sanpitak P. Epithelial-stromal junction of intraductal carcinoma of the breast. Cancer. 1970 Dec;26(6):1186–1198. doi: 10.1002/1097-0142(197012)26:6<1186::aid-cncr2820260603>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., Rønnov-Jessen L., Howlett A. R., Bissell M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Cardillo M. R., Hanby A., Stamp G. W. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol. 1992 Oct;23(10):1159–1166. doi: 10.1016/0046-8177(92)90034-z. [DOI] [PubMed] [Google Scholar]
- Riddelle K. S., Green K. J., Jones J. C. Formation of hemidesmosomes in vitro by a transformed rat bladder cell line. J Cell Biol. 1991 Jan;112(1):159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddelle K. S., Hopkinson S. B., Jones J. C. Hemidesmosomes in the epithelial cell line 804G: their fate during wound closure, mitosis and drug induced reorganization of the cytoskeleton. J Cell Sci. 1992 Oct;103(Pt 2):475–490. doi: 10.1242/jcs.103.2.475. [DOI] [PubMed] [Google Scholar]
- Rucklidge G. J., Edvardsen K., Bock E. Cell-adhesion molecules and metalloproteinases: a linked role in tumour cell invasiveness. Biochem Soc Trans. 1994 Feb;22(1):63–68. doi: 10.1042/bst0220063. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993 Apr 1;53(7):1503–1506. [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Sciubba J. J. Regeneration of the basal lamina complex during epithelial wound healing. J Periodontal Res. 1977 May;12(3):204–217. doi: 10.1111/j.1600-0765.1977.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Sakai L. Y., Burgeson R. E., Holbrook K. A. Ontogeny of structural components at the dermal-epidermal junction in human embryonic and fetal skin: the appearance of anchoring fibrils and type VII collagen. J Invest Dermatol. 1988 Apr;90(4):480–485. doi: 10.1111/1523-1747.ep12460951. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. R., Alvarez O. M., Bere E. W., Jr, Eaglstein W. H., Katz S. I. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J Invest Dermatol. 1981 Aug;77(2):240–243. doi: 10.1111/1523-1747.ep12480082. [DOI] [PubMed] [Google Scholar]
- Stanley J. R. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J. W., Chandler J. A. The fine structure of the normal, resting terminal ductal-lobular unit of the female breast. Virchows Arch A Pathol Anat Histol. 1976 Dec 27;372(3):205–226. doi: 10.1007/BF00433280. [DOI] [PubMed] [Google Scholar]
- Tannenbaum M., Weiss M., Marx A. J. Ultrastructure of the human mammary ductule. Cancer. 1969 Apr;23(4):958–978. doi: 10.1002/1097-0142(196904)23:4<958::aid-cncr2820230435>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Malone K. L., Dave K., Zhao S. M. Localization of the 230-kilodalton bullous pemphigoid antigen in cultured keratinocytes: formation of a prehemidesmosome. Exp Cell Res. 1991 Jun;194(2):238–247. doi: 10.1016/0014-4827(91)90360-7. [DOI] [PubMed] [Google Scholar]
- Verrando P., Blanchet-Bardon C., Pisani A., Thomas L., Cambazard F., Eady R. A., Schofield O., Ortonne J. P. Monoclonal antibody GB3 defines a widespread defect of several basement membranes and a keratinocyte dysfunction in patients with lethal junctional epidermolysis bullosa. Lab Invest. 1991 Jan;64(1):85–92. [PubMed] [Google Scholar]
- Watson R. J., Eyden B. P., Howell A., Sellwood R. A. Ultrastructural observations on the basal lamina in the normal human breast. J Anat. 1988 Feb;156:1–10. [PMC free article] [PubMed] [Google Scholar]
- Wetzels R. H., Robben H. C., Leigh I. M., Schaafsma H. E., Vooijs G. P., Ramaekers F. C. Distribution patterns of type VII collagen in normal and malignant human tissues. Am J Pathol. 1991 Aug;139(2):451–459. [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Daniel C. W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983 Jun;97(2):274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Burgeson R. E., Lunstrum G., Bruckner-Tuderman L., Reese M. J., Briggaman R. A. Epidermolysis bullosa acquisita antigen is the globular carboxyl terminus of type VII procollagen. J Clin Invest. 1988 Mar;81(3):683–687. doi: 10.1172/JCI113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., O'Keefe E. J., Prunieras M. Cutaneous wound healing: a model for cell-matrix interactions. J Am Acad Dermatol. 1985 Feb;12(2 Pt 2):420–433. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]