Abstract
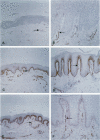
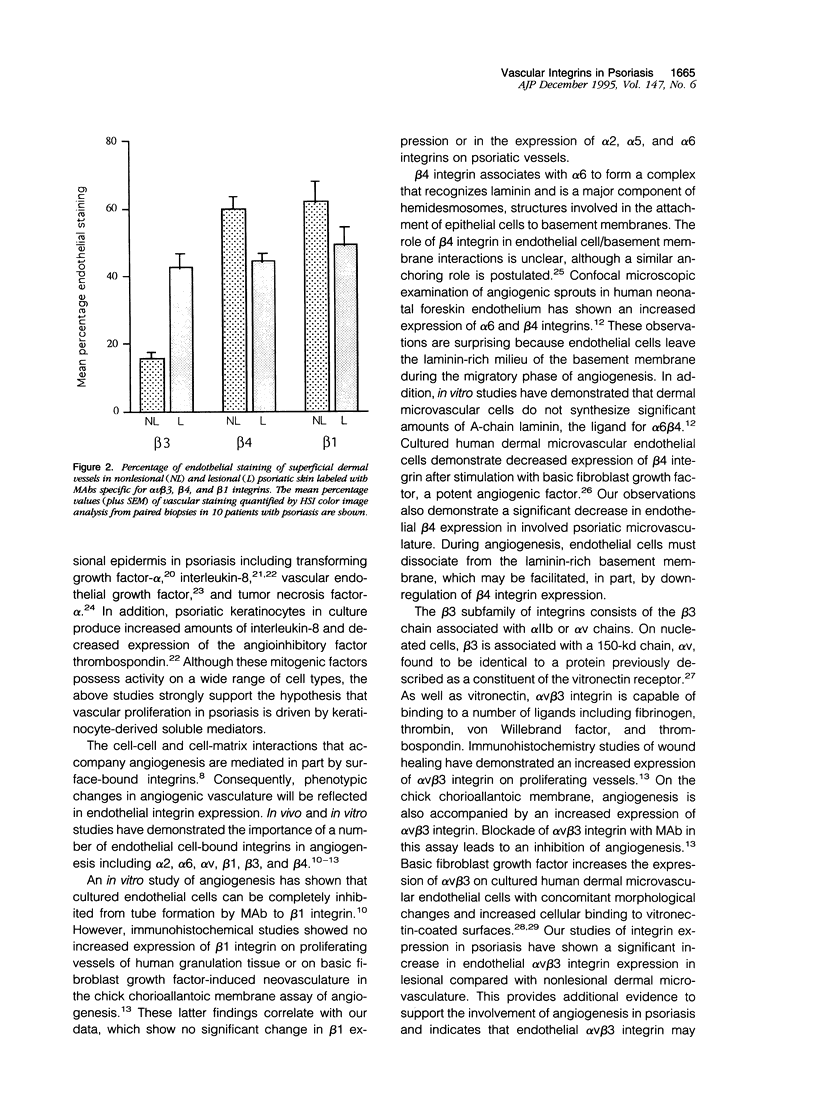
Considerable evidence indicates that microvascular changes observed in psoriasis are a result of vascular proliferation. A critical step in the sequence of events leading to neovascularization involves interactions between endothelial cells and extracellular matrix proteins mediated in part by the integrin family of adhesion molecules. A number of endothelial integrins have been shown to participate in neovascularization, including members of the beta 1, beta 3, and beta 4 subfamilies. To investigate the role of these integrins in psoriasis, specimens of lesional and nonlesional skin were taken from 10 patients with active, untreated plaque disease. Vascular endothelium was labeled with monoclonal antibodies specific for alpha 2, alpha 5, alpha 6, beta 1, av beta 3, and beta 4 integrins. The use of image analysis permitted quantification of immunoperoxidase staining and comparison of endothelial labeling in lesional and nonlesional skin. There was a significant increase in endothelial staining of av beta 3 integrin in lesional compared with nonlesional skin, both in superficial and deep vasculature. In contrast, there was a significant decrease in endothelial beta 4 staining in lesional compared with nonlesional superficial dermal vessels, alpha 2, alpha 5, alpha 6, and beta 1 staining showed no significant difference between the two groups. These results demonstrate an important role of av beta 3 and beta 4 integrins in the microvascular changes of psoriatic lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991 Mar;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- Allen M. H., Markey A. C., MacDonald D. M. The development of a reproducible immunocytochemical technique for demonstrating colocalized cutaneous antigens. Am J Dermatopathol. 1991 Jun;13(3):221–227. doi: 10.1097/00000372-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Atherton D. J., Wells R. S., Laurent M. R., Williams Y. F. Razoxane (ICRF 159) in the treatment of psoriasis. Br J Dermatol. 1980 Mar;102(3):307–317. doi: 10.1111/j.1365-2133.1980.tb08145.x. [DOI] [PubMed] [Google Scholar]
- Auerbach W., Auerbach R. Angiogenesis inhibition: a review. Pharmacol Ther. 1994 Sep;63(3):265–311. doi: 10.1016/0163-7258(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Barker J. N. The pathophysiology of psoriasis. Lancet. 1991 Jul 27;338(8761):227–230. doi: 10.1016/0140-6736(91)90357-u. [DOI] [PubMed] [Google Scholar]
- Barton S. P., Abdullah M. S., Marks R. Quantification of microvascular changes in the skin in patients with psoriasis. Br J Dermatol. 1992 Jun;126(6):569–574. doi: 10.1111/j.1365-2133.1992.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Bauer J., Margolis M., Schreiner C., Edgell C. J., Azizkhan J., Lazarowski E., Juliano R. L. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992 Dec;153(3):437–449. doi: 10.1002/jcp.1041530302. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Sibley J. Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol. 1982 Jan;78(1):12–17. doi: 10.1111/1523-1747.ep12497850. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Detmar M., Brown L. F., Claffey K. P., Yeo K. T., Kocher O., Jackman R. W., Berse B., Dvorak H. F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994 Sep 1;180(3):1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Enenstein J., Kramer R. H. Confocal microscopic analysis of integrin expression on the microvasculature and its sprouts in the neonatal foreskin. J Invest Dermatol. 1994 Sep;103(3):381–386. doi: 10.1111/1523-1747.ep12395390. [DOI] [PubMed] [Google Scholar]
- Ettehadi P., Greaves M. W., Wallach D., Aderka D., Camp R. D. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994 Apr;96(1):146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Gamble J. R., Matthias L. J., Meyer G., Kaur P., Russ G., Faull R., Berndt M. C., Vadas M. A. Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol. 1993 May;121(4):931–943. doi: 10.1083/jcb.121.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemaaijer R., Koolwijk P., le Clercq L., de Vree W. J., van Hinsbergh V. W. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993 Dec 15;296(Pt 3):803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin Cancer Biol. 1992 Apr;3(2):57–63. [PubMed] [Google Scholar]
- Kennel S. J., Godfrey V., Ch'ang L. Y., Lankford T. K., Foote L. J., Makkinje A. The beta 4 subunit of the integrin family is displayed on a restricted subset of endothelium in mice. J Cell Sci. 1992 Jan;101(Pt 1):145–150. doi: 10.1242/jcs.101.1.145. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994 Sep;8(12):929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Varani J., Dixit V. M., Polverini P. J. Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am J Pathol. 1994 Apr;144(4):820–828. [PMC free article] [PubMed] [Google Scholar]
- Norrby K. Cyclosporine is angiostatic. Experientia. 1992 Dec 1;48(11-12):1135–1138. doi: 10.1007/BF01948007. [DOI] [PubMed] [Google Scholar]
- Pellegrini G., De Luca M., Orecchia G., Balzac F., Cremona O., Savoia P., Cancedda R., Marchisio P. C. Expression, topography, and function of integrin receptors are severely altered in keratinocytes from involved and uninvolved psoriatic skin. J Clin Invest. 1992 Jun;89(6):1783–1795. doi: 10.1172/JCI115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Identification of C5ades arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest Dermatol. 1986 Jul;87(1):53–58. doi: 10.1111/1523-1747.ep12523566. [DOI] [PubMed] [Google Scholar]
- Sepp N. T., Cornelius L. A., Romani N., Li L. J., Caughman S. W., Lawley T. J., Swerlick R. A. Polarized expression and basic fibroblast growth factor-induced down-regulation of the alpha 6 beta 4 integrin complex on human microvascular endothelial cells. J Invest Dermatol. 1995 Feb;104(2):266–270. doi: 10.1111/1523-1747.ep12612807. [DOI] [PubMed] [Google Scholar]
- Sepp N. T., Li L. J., Lee K. H., Brown E. J., Caughman S. W., Lawley T. J., Swerlick R. A. Basic fibroblast growth factor increases expression of the alpha v beta 3 integrin complex on human microvascular endothelial cells. J Invest Dermatol. 1994 Sep;103(3):295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- Swerlick R. A., Brown E. J., Xu Y., Lee K. H., Manos S., Lawley T. J. Expression and modulation of the vitronectin receptor on human dermal microvascular endothelial cells. J Invest Dermatol. 1992 Dec;99(6):715–722. doi: 10.1111/1523-1747.ep12614207. [DOI] [PubMed] [Google Scholar]
- Tenchini M. L., Adams J. C., Gilberty C., Steel J., Hudson D. L., Malcovati M., Watt F. M. Evidence against a major role for integrins in calcium-dependent intercellular adhesion of epidermal keratinocytes. Cell Adhes Commun. 1993 May;1(1):55–66. doi: 10.3109/15419069309095681. [DOI] [PubMed] [Google Scholar]
- Wolf J. E., Jr Angiogenesis in normal and psoriatic skin. Lab Invest. 1989 Aug;61(2):139–142. [PubMed] [Google Scholar]