Abstract
Marrow stromal cells from wild-type mice were infused into transgenic mice that had a phenotype of fragile bones resembling osteogenesis imperfecta because they expressed a human minigene for type I collagen. In mice that were irradiated with potentially lethal levels (700 cGy) or sublethal levels (350 cGy), DNA from the donor marrow stromal cells was detected consistently in marrow, bone, cartilage, and lung either 1 or 2.5 mo after the infusions. The DNA also was detected but less frequently in the spleen, brain, and skin. There was a small but statistically significant increase in both collagen content and mineral content of bone 1 mo after the infusion. Similar results were obtained with infusion of relatively large amounts of wild-type whole marrow cells into the transgenic mice. In experiments in which male marrow stromal cells were infused into a female osteogenesis imperfecta-transgenic mouse, fluorescense in situ hybridization assays for the Y chromosome indicated that, after 2.5 mo, donor male cells accounted for 4–19% of the fibroblasts or fibroblast-like cells obtained in primary cultures of the lung, calvaria, cartilage, long bone, tail, and skin. In a parallel experiment in which whole marrow cells from a male mouse were infused into a female immunodeficient rag-2 mouse, donor male cells accounted for 4–6% of the fibroblasts or fibroblast-like cells in primary cultures. The results support previous suggestions that marrow stromal cells or related cells in marrow serve as a source for continual renewal of cells in a number of nonhematopoietic tissues.
In addition to hematopoietic stem cells, bone marrow contains cells that meet most of the criteria for stem cells of nonhematopoietic tissues (1–10). The cells from rodents and rabbits originally were referred to as colony-forming units fibroblasts (1, 2) because they adhered to tissue culture plastic and formed colonies of fibroblast-like cells. More recently, they have been referred to either as mesenchymal stem cells because of their ability to differentiate into cells that can be defined roughly as mesenchymal (7) or as marrow stromal cells (MSCs) because they appear to arise from a complex array of supporting structures found in marrow and because they can provide a feeder layer for cultured hematopoietic stem cells (10–12). Clones of MSCs have been difficult to generate (13–18), and therefore most experiments with MSCs have been carried out with heterogeneous populations that are isolated with the simple first protocol devised by Friedenstein et al. (1, 4) in which the cells are recovered by their tendency to adhere tightly to plastic culture dishes. Under appropriate culture conditions that are in part species-dependent, MSCs can differentiate into adipocytes, chondrocytes, osteoblast-like cells, and myotubes (1–18). Also, after implantation in chambers with porous membranes or on ceramic cubes, the cells form fibrous tissue, cartilage, and bone in vivo. Some reports (19–24) suggested that MSCs do not engraft in marrow after systemic infusion, but other reports described successful marrow engraftment (2, 25–30). Many of the negative experiments may be explained by species differences in the animal models, the conditions used for marrow ablation, the number of cells infused, the protocols used to prepare the cells, or the markers used to identify the donor cells (10).
We reported (30) that 1 day after infusion of MSCs containing a marker type I collagen gene into irradiated isogenic mice, the donor MSCs accounted for 0.002% or less of the cells in the recipient mice. However, after 1 and 5 mo, progeny of the donor MSCs accounted for 1–10% of the cells in both hematopoietic tissues and nonhematopoietic tissues such as bone, cartilage, and lung. Moreover, the progeny of MSCs in bone and cartilage appeared to acquire the phenotypes of the tissues they entered because mRNA from the marker type I collagen tissue was observed in bone that is rich in type I collagen but not in cartilage, a tissue that does not contain type I collagen. Here, we extended our previous observations by infusion of either wild-type MSCs or wild-type whole marrow cells (WMCs) into osteogenesis imperfecta-transgenic (OI-transgenic) mice that develop a phenotype of brittle bones because they express a mini-COL1A1 gene for type I procollagen (31–34).
METHODS
Transgenic Mice.
The recipient mice were OI-transgenic mice expressing a human mini-COL1A1 gene and were from the inbred FVB/N line we defined as Line 73 (31–34). Because of defective teeth, they were maintained on a combination of pelleted and powdered chow. To identify transgenic pups, toes were cut, and DNA was extracted for PCR assay of the transgene (30). At the age of 3 wk, the OI-transgenic pups (≈9 g) were irradiated either with a 137Cs irradiator (Atomic Energy, Ottawa) at 116 cGy/min or an x-ray irradiator (EXL14; Mitsubishi Oil Chemicals, Tokyo) at 200 cGy/min. Preliminary trials established that the potentially lethal dose in the transgenic mice was 700 cGy or ≈200 cGy less than for normal littermates (30). The irradiation with 700 cGy was in two equal amounts administered after a 4-h interval; lower levels were in a single administration. The mice received the first i.p. infusion of mixtures of MSCs and WMCs, or WMCs alone, 1–2 h after the last irradiation. The T and B lymphocyte-deficient rag-2 mice were obtained from Taconic Farms.
MSCs and WMCs.
Donor marrow was obtained either from wild-type FVB/N mice or OI-transgenic mice. Tibias and femurs were dissected from 8- to 12-week-old mice, the ends of the bones were cut, and marrow was flushed out with 2 ml of ice-cold α-MEM (Sigma) containing 10% (vol/vol) fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA) by using a needle and syringe. FBS lots were selected for low endotoxin levels and were prescreened after heat inactivation at 55°C for 1 h to result in the maximal number of colonies formed by WMCs grown in agar for 1 wk (35). The same two lots were used for all experiments. The pooled marrow cells were dispersed by agitation through a syringe, and nucleated cells were counted electronically (Model ZM or Multisizer II, Coulter). The samples were diluted in concentrations 0.1–2 × 107 cells per ml in α-MEM and 10% FBS. They were used directly as WMCs.
To obtain MSCs, 10–50 × 106 nucleated WMCs, in 25 ml of α-MEM and 10% FBS, were plated on 75-cm2 culture flasks (Falcon; Becton Dickinson). After 3 days, approximately one-half of the nonadherent cells was removed by replacing one-half of the medium. Again, one-half of the medium was replaced with fresh medium on days 10 and 17. The cultures were harvested after they reached confluency at 20–22 days. Before harvesting, the medium was removed, and the cells were washed with PBS. The cells were then incubated in 4 ml of 0.25% trypsin and 0.5 mM EDTA (GIBCO/BRL) at 37°C for 20 min. The digestion was stopped by adding 8 ml of culture medium. The detached cells that largely came from the centers of foci were taken as MSCs; the remaining adherent cells were discarded. The MSCs were recovered by centrifugation at 500 × g for 15 min and suspended at a concentration of 0.1–2 × 107 nucleated cells per milliliter of α-MEM and 10% FBS. Mixtures of MSCs and WMCs, or suspensions of WMCs alone, were injected i.p. into the recipient 3-wk-old irradiated mice in vol of 0.2–0.4 ml. In most experiments, the i.p. injections were repeated every other day for a total of three or four injections.
The Osteogenic Potential of MSCs.
WMCs were seeded in 4-cm2 wells at a density of 5 × 106 cells per well. After 3 days, the nonadherent cells were removed, and the medium was changed every third day thereafter. For alkaline phosphatase (ALP) activity and total protein determination, the adherent cells were washed twice with PBS and lysed for 10 min at room temperature with 15 mM Tris⋅HCl buffer (pH 7.4) containing 1 mM ZnCl2, 1 mM MgCl2, and 1% Triton X-100. Aliquots of cell lysates were mixed with assay buffer containing 7 mM p-nitrophenol phosphate (Sigma), incubated for 20 min at room temperature, and then assayed for A at 410 nm. Total protein content was assayed with a commercial kit (DC Protein Assay Kit, Bio-Rad). ALP activity was expressed as nanomoles of p-nitrophenol per min/μg protein assuming that A at 410 nm is equivalent to 64 nanomoles of p-nitrophenol.
PCR Assays.
For isolation of DNA, a 2- to 10-mm2 piece of tissue was incubated at 55°C overnight in 0.1–0.2 ml of 10 mM Tris⋅HCl (pH 8.0), 50 mM KCl, 5 mM MgCl2, 0.5 mg/ml gelatin, 1% Brij-35 and 0.4 mg/ml proteinase K. After digestion, the DNA was denatured at 100°C for 8 min and cooled on ice. Aliquots of 5 μl were used in the PCR assay, and the remainder was stored at −80°C.
For PCR assays of the human mini-COL1A1 gene, the 5′ primer (BS49, CAGTCGTCGGAGCAGACGGGAGTTT) had a sequence that hybridized to the same sequence in exon 1 of both the human COL1A1 gene and endogenous mouse Col1a1 gene (30, 33). It was labeled with 32P (30). Two different 3′ primers were used. One 3′ primer consisted of sequences (BS47, ACTCCCCAGAGTTTGGAACTTACTGTC) targeted to a downstream sequence of exon 1 in the human COL1A1 gene. The second 3′ primer (BS48, ACTCCCAAAAGTTTGGGACTTACTGTC) that differed by only three nucleotides was targeted to the homologous sequence in the mouse Col1a1 gene. Because of natural deletions of 25 nt in the 5′ nontranslated region of the mouse Col1a1 gene, the product from the human gene was 257 bp, and the product from the mouse gene was 232 bp. The conditions for PCR were 1 min at 94°C and 2 min at 67°C for a total of 31 cycles. Ten microliters of the reaction product was denatured at 100°C for 8 min and electrophoresed on a 7% polyacrylamide gel containing 6 M urea. The gel was fixed, dried, and assayed by exposing the gel to a phosphostimulable storage plate (400S Phosphorimager, Molecular Dynamics).
Assays of Collagen and Mineral Content.
Freshly dissected humeri were hydrolyzed in 2 ml of 6 M HCl at 100°C overnight, and the hydroxyproline content was assayed (36). The collagen content was calculated from the hydroxyproline values by assuming a 10.11% content (37). To determine the mineral content, freshly dissected femurs were ash dried in aluminum foil at 600°C for 18 h and weighed.
Fluorescense In Situ Hybridization (FISH) Assays and Immunofluorescence on Primary Cultures.
Tissue samples were cultured in either plastic or glass 4-chamber slides (Lab-Tek Chamber Slide system, Fisher Scientific) in 0.7 ml of α-MEM and 10% FBS. MSCs were prepared as described above. Samples of long bone were washed thoroughly free of marrow and then crushed. Samples of cartilage were obtained by dissecting the xiphoid free of adjacent tissues and perichondrium. All of the tissues were thoroughly washed with α-MEM and FCS and then minced. Medium was replaced every 3–5 days, and the cells were grown for 4–10 days. The probe for the Y chromosome was a 1.6-kb EcoRI fragment (38) that was labeled with biotin by using a commercial kit (Bionick Labeling System, GIBCO/BRL) and was used for FISH assay with fluorescein isothiocyanate-avidin (39).
The primary cultures also were stained with rabbit polyclonal antibodies to mouse type I collagen (Biodesign International, Kennebunk, ME) or with a rat mAb to the mouse Mac-1 α-chain (antimouse CD11b/integrin αM-chain, PharMingen). The secondary antibodies were the Fab fragment of goat–antirabbit IgG (Cappel) or rabbit–antirat IgG (PharMingen), both labeled with fluorescein isothiocyanate. Control experiments were carried out with species- and isotype-matched control antibodies at equimolar concentrations.
RESULTS
Osteogenic Potential of MSCs from Wild-Type and OI-Transgenic Mice.
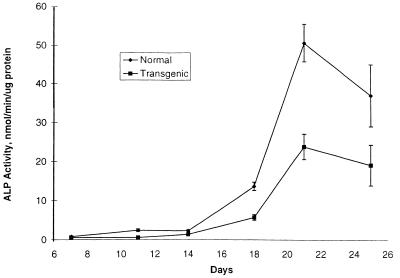
Previous observations indicated that normal skin fibroblasts had a growth advantage over some skin fibroblasts from patients with OI (see ref. 40), apparently because the OI fibroblasts accumulated incorrectly folded procollagen in the cisternae of the rough endoplasmic reticulum. Here, no apparent difference in growth rates was seen between MSCs from wild-type mice and OI-transgenic mice either in comparisons of individual cultures or in cultures containing mixtures of the two populations (not shown). However, the levels of ALP activity in 2-wk cultures of MSCs from OI-transgenic mice were approximately one-half of the levels observed with MSCs from control littermates (Fig. 1). Staining of the cultures for mineral deposits with Alizarin red confirmed the impression that MSCs from the OI-transgenic mice had decreased osteogenic potential (not shown).
Figure 1.
ALP activity in mouse MSCs. Cells were cultured, and ALP activity per μg protein was assayed as described in text. Values are means ± SD (n = 3).
Infusion of Wild-Type MSCs into OI-Transgenic Mice.
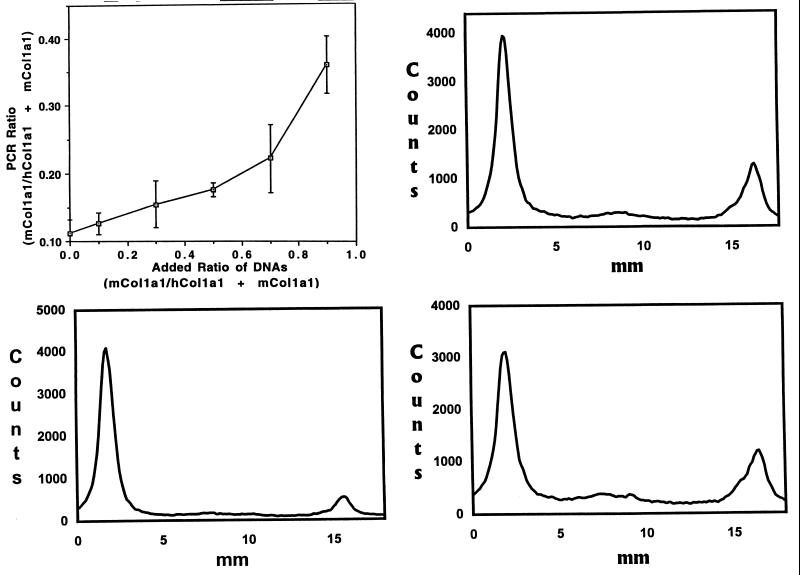
Wild-type MSCs without a marker gene were infused i.p. into OI-transgenic mice that were marrow ablated by x-ray irradiation. To detect progeny of infused wild-type MSCs, a PCR assay was used to assess the relative levels of the endogenous mouse Col1a1 gene and the human mini-COL1A1 in tissues (30). Standard curves were prepared with mixtures of tail DNA from wild-type and OI-transgenic mice. The assay was shown to be linear over a broad range in mixtures containing high ratios of wild-type-to-OI-transgenic DNA (30). However, standard curves were nonlinear with mixtures containing low ratios in the range required to detect appearance of wild-type DNA in the OI-transgenic mice (Fig. 2, Upper Left), apparently because of the high copy number of the transgene (Fig. 2, Lower Left). Accordingly, the assay was used here only in a semiquantitative manner. The PCR assays indicated that significant levels of donor DNA were present in a variety of tissues from most irradiated OI-transgenic mice (Fig. 2 and Table 1). The donor DNA was detected in marrow from all of the mice except two of seven that were irradiated with the lowest dose of 175 cGy/min. In one-half or more of the mice, it was detected in spleen (55%), bone (82%), lung (69%), cartilage (70%), and brain (50%). It was detected in skin of only one of nine mice (11%), perhaps because the skin of mice consists largely of epidermis and hair follicles. Within the limits of the experiment, there were no apparent differences in mice assayed at 1 or 2.5 mo after the infusions. Additionally, there were no apparent differences with a tenfold range of number of MSCs infused (from ≈0.7 to 5.8 × 108/kg). Also, there were no apparent differences with reduction in the dose used for marrow ablation from the potentially lethal level of 700 to 350 cGy. In one experiment in which the irradiation was reduced to 175 cGy, the values for the presence of donor DNA in tissues were more variable than with higher doses of irradiation.
Figure 2.
PCR assay for ratio of DNA from the endogenous murine Col1a1 gene (mCol1a1) to the mCol1a1 gene plus the human mini COL1A1 gene (hCol1a1). (Upper Left) Standard curve prepared with mixtures of DNA from tails of wild-type and OI-transgenic mice. Values are means ± 2 SD of three separate assays in duplicate (n = 6). (Lower Left) Assay of tail DNA from an OI-transgenic mouse. The PCR products were separated by using 7% PAGE containing 7 M urea, and the image on a phosphor storage plate was scanned. The human mini-COL1A1 is present in a high copy number (≈100). (Upper Right) Assay of marrow DNA from an OI-transgenic mouse that was marrow-ablated with 700 cGy/min and infused with 12 × 106 wild-type MSCs 1 mo earlier. (Lower Right) Assay of bone DNA from the same OI-transgenic mouse as shown in Upper Right.
Table 1.
Detection of progeny of wild-type MSCs infused into OI-transgenic mice
| Dose, cGy | Duration, mo | Donor MSCs, ×106* | Mice with positive tissues/total†
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Marrow | Spleen | Bone | Lung | Cartilage | Brain | Skin | |||
| 700 | 1 | 0.7 | 3/3 | 3/3 | 2/3 | 2/3 | 2/3 | 1/3 | 0/3 |
| 700 | 1 | 5.8 | 4/4 | 0/4 | 4/4 | 2/4 | 4/4 | 1/4 | |
| 700 | 1 | 12 | 2/2 | 2/2 | 2/2 | 2/2 | 1/2 | ||
| 700 | 2.5 | 3.1 | 6/6 | 6/6 | |||||
| 700 | 2.5 | 5.8 | 3/3 | 3/3 | 2/2 | ||||
| 350 | 1 | 1.2 | 4/4 | 2/4 | 3/4 | 3/4 | 4/4 | 3/4 | 0/4 |
| 350 | 1 | 5.8 | 2/2 | 0/2 | 2/2 | 2/2 | 1/2 | ||
| 350 | 2.5 | 3.1 | 7/7 | ||||||
| 350 | 2.5 | 5.8 | 2/2 | 3/3 | 3/3 | ||||
| 175 | 1 | 1.2 | 5/7 | 5/7 | 3/7 | 2/2 | 0/7 | 4/7 | 1/2 |
| TOTALS | 38/40 | 12/22 | 28/34 | 9/13 | 19/27 | 11/22 | 1/9 | ||
| (95%) | (55%) | (82%) | (69%) | (70%) | (50%) | (11%) | |||
Before infusion, donor wild-type MSCs were mixed with 2 to 15 × 106 nucleated WMCs from an OI-transgenic mouse to provide a source of hematopoietic precursors. In most experiments the ratio of OI-MSCs to WMCs varied from 1:0.17 to 1:2.8.
Tissues in which values for PCR assay (see Fig. 2) were equal to or greater than mean plus 2 × SD for untreated OI transgenic mice (mean = 0.14 ± 0.015 SD; n = 25). Reference to the standard curve (Fig. 2) suggested that values equal to or greater than the mean plus 2 × SD reflected samples in which 30% or more of the cells were derived from donor MSCs.
Similar results were obtained with infusion of relatively large amounts of wild-type WMCs (from 17 to 84 × 108/kg) into the OI-transgenic mice (Table 2). In most of the mice, the donor DNA was detected in marrow (98%) and cartilage (98%). In over one-half of the mice, it was detected in spleen (70%) and bone (60%). It was detected somewhat less consistently in brain (42%). Reducing the irradiation from 700 to 350 cGy or to 175 cGy appeared to have little effect.
Table 2.
Detection of progeny of wild-type WMCs infused into OI-transgenic mice
| Dose, cGy | Duration, mo | Donor WMCs, ×106 | Mice with positive tissues/total*
|
||||
|---|---|---|---|---|---|---|---|
| Marrow | Spleen | Bone | Cartilage | Brain | |||
| 700 | 1 | 17 | 7/7 | 5/7 | 6/7 | 7/7 | 2/6 |
| 700 | 2.5 | 84 | 6/7 | 7/7 | 4/7 | 7/7 | |
| 350 | 1 | 16 | 3/3 | 1/3 | 3/3 | 2/2 | |
| 350 | 1 | 17 | 7/7 | 5/6 | 5/6 | 6/6 | 4/6 |
| 350 | 2.5 | 84 | 6/6 | 5/6 | 0/6 | 6/6 | |
| 175 | 1 | 17 | 7/7 | 7/7 | 7/7 | 7/7 | 2/7 |
| 175 | 2.5 | 84 | 7/7 | 0/7 | 1/7 | 6/7 | |
| TOTALS | 43/44 | 30/43 | 26/43 | 41/42 | 8/19 | ||
| (98%) | (70%) | (60%) | (98%) | (42%) | |||
Effects of Donor Wild-Type MSCs and WMCs on Collagen Content and Mineral Content of Bone in OI-Transgenic Mice.
Humeri from a series of OI-transgenic mice that received wild-type MSCs or wild-type WMCs were assayed 1 mo after infusion of the donor cells (Table 3). In OI-transgenic mice that received wild-type MSCs, there was a small but statistically significant increase in both the collagen and mineral content of bone. In OI-transgenic mice that received wild-type WMCs, there was a small but statistically significant increase in the collagen content of bone.
Table 3.
Collagen and mineral content of bone (humerus) in OI-transgenic mice one mo after infusion of wild-type MSCs or WMCs
| Donor cells* | Donor mice | Collagen content mg ± SD | Mineral content mg ± SD |
|---|---|---|---|
| WMCs | OI transgenic | 2.11 ± 0.52 | 7.78 ± 2.12 |
| (n = 25) | (n = 21) | ||
| MSCs | Wild-type | 2.41 ± 0.37† | 9.40 ± 1.68‡ |
| (n = 19) | (n = 20) | ||
| WMCs | Wild-type | 2.33 ± 0.48† | 8.26 ± 2.13 |
| (n = 22) | (n = 20) |
OI-transgenic mice were irradiated with 175, 350, or 700 cGy as in Tables 1 and 2. Values for irradiated wild-type mice: Collagen = 3.59 mg ± 0.38 SD (n = 14); mineral = 11.45 mg ± 1.44 SD (n = 14).
Student’s t test for difference from controls that received WMCs from OI-transgenic mice, P = 0.03
Student’s t test for difference from controls, P = 0.01
FISH Assays on Primary Cultures.
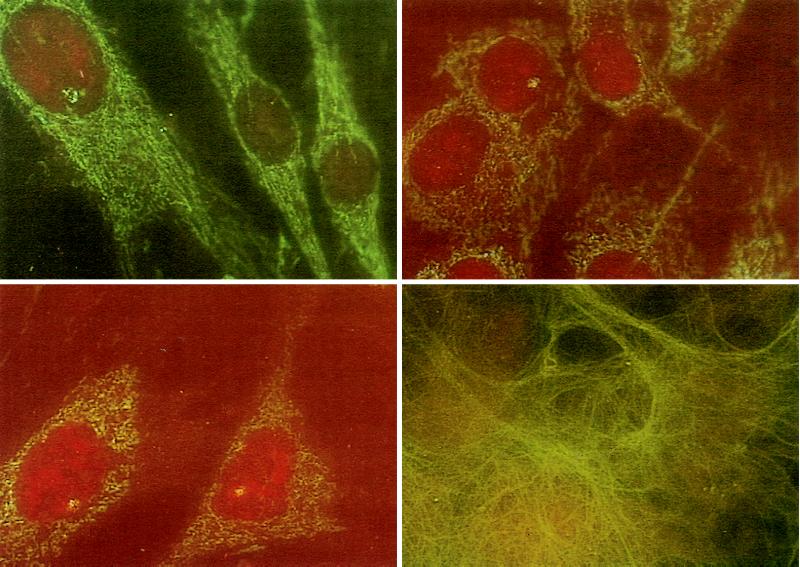
To follow further the tissue fate of infused MSCs, wild-type MSCs from isogenic males together with WMCs from OI-transgenic mice were infused i.p. into four female OI-transgenic mice after marrow ablation. After 2.5 mo, tail DNA was assayed by using PCR for a decreased ratio of the endogenous mini-COL1A1 gene to the Col1a1 gene (Fig. 2). The recipient mouse with the largest increase in the ratio then was used to prepare primary cell cultures. The Y chromosome was detected readily by FISH analyses of primary cultures from a number of tissues (Fig. 3). The values for cells containing the Y chromosome varied from 4% in cells cultured from skin to 19% in cells cultured from lung (Table 4). However, no male cells were detected in primary cultures from the heart and aorta. Most of the cells in the cultures had the morphology of fibroblasts. The fibroblast-like cells synthesized large amounts of type I collagen (Fig. 3, Lower Right). Staining the same culture with an antibody to Mac-1 (not shown) indicated that 0.2–1.2% of the cells were macrophages. The macrophages were morphologically distinguishable from fibroblasts.
Figure 3.
FISH assay for Y chromosome and immunofluorescence for type I collagen in primary cultures of cells. Wild-type MSCs (11 × 106) from an isogenic male mouse together with WMCs (2 × 106) from an OI-transgenic mouse were infused into a 3-week-old female OI-transgenic mouse that was marrow ablated (Experiment 1 in Table 4). After 2.5 mo primary cultures were prepared. (Upper Left) Primary cultures from skin assayed by FISH. Cell at Upper Left contains a Y chromosome. (Upper Right) Primary culture of tail from same mouse. Cell in Upper Left contains the Y chromosome. (Lower Left) Primary culture of tail from a control male mouse. (Lower Right) Cells from same primary culture of skin as in Upper Left stained with antibodies to type I collagen.
Table 4.
FISH assay for Y chromosome on primary cultures of female mice infused with wild-type male MSCs or male WMCs
| Source of primary cultures | Cells with Y chromosome*
|
|
|---|---|---|
| Experiment 1, %† | Experiment 2, %‡ | |
| Lung | 19 | NA§ |
| Calvaria | 15 | 4 |
| Cartilage | 8 | NA |
| Long bone | 7 | 6 |
| Tail | 6 | 5 |
| Bone marrow (MSCs) | 5 | 5 |
| Skin | 4 | NA |
| Aorta | 0 | NA |
| Heart | 0 | NA |
Values from FISH assays of 200 cells in the primary cultures.
Wild-type MSCs (11 × 106) from an isogenic male mouse (FVB) together with WMCs (2 × 106) from an OI-transgenic mouse were infused i.p. into a 3-wk-old OI-transgenic mouse after irradiation with 700 cGy. Primary cultures were prepared 2.5 mo later.
WMCs (6.7 × 106) from a male FVB mouse were infused i.p. into a 3-wk-old female rag-2 mouse after marrow ablation with 700 cGy. Primary cultures were prepared 3 mo later.
NA, not assayed because tissues did not generate adequate primary cultures.
In a parallel experiment, WMCs from a male FVB mouse were infused i.p. into a 3-wk-old female immunodeficient rag-2 mouse (41) after marrow ablation with 700 cGy. The Y chromosome was present in 4–6% of the cells obtained in primary cultures (Table 4).
DISCUSSION
Recently, several independent reports confirmed the observation (30) that systemically infused MSCs can repopulate a number of nonhematopoietic tissues. Some of the reports also indicated that the cells acquire the cellular phenotypes of the tissues they repopulate. Keating et al. (42, 43) isolated MSCs, stably transfected the cells with a retrovirus containing the gene for human factor IX, and then infused the cells into immunodeficient SCID mice that did not undergo marrow ablation. Two months later, the donor MSCs accounted for 0.2–2.3% of the cells in the liver, thymus, and lung. Hou et al. (44) obtained MSCs from transgenic mice that expressed a chloramphenicol acetyl transferase gene driven by a promoter for the osteocalcin gene. After systemic infusion of MSCs from the transgenic mice, they detected chloramphenicol acetyl transferase activity in bone of recipient mice. Nilsson et al. (45) isolated MSCs from male mice and infused them into female mice that did not undergo marrow ablation. They detected cells with the Y chromosome among encapsulated osteocytes within bone lacunae and as flattened bone lining cells in the periosteum of recipient mice. Onyia et al. (46) transfected rat MSCs with a retrovirus that expressed a neomycin resistance gene and a gene for β-galactosidase. After surgical ablation of marrow from a femur of a mature rat, they injected the transfected MSCs into the marrow space. Five to 6 days later, they detected the marker genes in osteoblasts cultured from the adjacent bone. Also, they detected cells that stained for β-galactosidase among osteoblasts on trabecular bone surfaces, among terminally differentiated osteocytes within bone, and among a few chondrocytes in the healing cartilage plate. In their experiments, Onyia et al. (46) found that MSCs obtained from metaphyses in which endosteal cells are proliferating actively were far more effective in repopulating bone than MSCs from diaphyses in which endosteal cells are normally less proliferative. Nakagawa et al. (47) injected [3H]thymidine-labeled MSCs i.p. into rats with type II collagen-induced arthritis. They detected the labeled cells within joint cavities and within sublining layers of proliferating synovial tissue. They suggested that some of the cells had migrated from the marrow through narrow channels passing through to the articular surfaces of the joints. In related experiments with infusions of WMCs instead of MSCs, Eglitis and Mezey (48) demonstrated that marrow-derived cells from male mice were recovered both as microglial and astroglial cells in the brains of adult female mice.
Here, MSCs isolated from OI-transgenic mice by their adherence to tissue culture plastic did not have a growth advantage over control MSCs. However, the MSCs from the OI-transgenic mice had a reduced tendency to differentiate spontaneously into osteoblast-like cells synthesizing ALP and depositing mineral in culture. Therefore, the results suggested that wild-type MSCs that were infused into OI-transgenic mice may have an advantage in replacing bone cells in the recipient mice. After 3-week-old OI transgenic mice were marrow-ablated and infused with wild-type MSCs, a semiquantitative assay for the ratio of the endogenous Col1a1 and mini-COL1A1 genes indicated that progeny of the MSCs accounted for a considerable number of the cells in a series of nonhematopoietic tissues, including bone, cartilage, and, to a lesser degree, spleen, brain, and skin. Similar results were obtained with infusion of large numbers of wild-type WMCs. The effects on the bone phenotype of the OI-transgenic mice were small, but there were statistically significant increases in bone content of both collagen and mineral in mice infused with MSCs. Also, there were small but statistically significant increases in the bone collagen in mice infused with WMCs. The lack of larger effects on the bone phenotype may be explained by the relatively short time period of 1 mo after the infusions. Assaying the effects on phenotype for longer than 1 mo in the OI-transgenic mice used here will require more extensive studies, primarily because the bones are more fragile and have a decreased collagen content up to 1.5 mo of age and at 24 mo but are indistinguishable from wild-type littermates at 6 mo (34).
In initial experiments, we were unable to follow the cellular fate of the infused cells by using MSCs from one line of transgenic mice expressing lacZ driven by a promoter for a gene for type I collagen (49) or from a second line of transgenic mice expressing lacZ driven by a promoter for a gene for the α2 chain of type XI collagen (S.-W. Li, M. Arita, and D.J.P., unpublished work), apparently because of a growth disadvantage or immune responses encountered by cells expressing β-galactosidase (not shown). Therefore, we infused wild-type MSCs from male mice into female OI-transgenic mice or wild-type WMCs from male mice into female immunodeficient rag-2 mice (41). FISH assays for the Y chromosome on primary cultures indicated that progeny of the infused MSCs or WMCs accounted for 4–19% of the cells in a number of nonhematopoietic tissues. The FISH assays may have either over- or underestimated the degree of cell replacement because the primary cultures are probably not representative of the tissue as a whole. However, the results established that the donor cells or progeny of the donors cells were recovered as primary fibroblasts or as fibroblast-like cells that are regularly obtained by spontaneous differentiation in culture of osteoblasts, chondrocytes, and other cell types.
One concern about experiments involving systemic infusion of MSCs isolated by their adherence to plastics is whether the cells that engraft into nonhematopoietic tissues are macrophages or macrophage-derived cells. The concern is particularly pertinent to experiments with murine MSCs because macrophages are present in relatively large numbers during the first few days of culture of murine MSCs (50). However, the presence of macrophages or macrophage precursors cannot explain the recovery of progeny of MSCs as fibroblasts in primary cultures from recipient animals as observed here or as osteoblasts and osteocytes in lacunae of bone as observed by others (44–46). Also, the presence of macrophages probably cannot explain the appearance of donor cells as phenotypically stained astroglial cells in brain after systemic infusion of WMCs (48). Therefore, it appears that progenitor cells in marrow serve as a source for continual renewal of cells in a number of nonhematopoietic tissues.
ABBREVIATIONS
- OI-transgenic mice
transgenic mice that had a phenotype of fragile bones resembling osteogenesis imperfecta because they expressed a human mini-COL1A1 containing a large in-frame deletion
- MSCs
marrow stromal cells that are isolated as the subpopulation of marrow cells that adhere to plastic and are cultured to confluency for ≈3 wk
- WMCs
whole marrow cells
- FBS
fetal bovine serum
- ALP
alkaline phosphatase
- FISH
fluorescense in situ hybridization
- cGy
centigray
References
- 1.Friedenstein A J, Gorskaja U, Kulagina N N. Exp Hematol (Charlottesville, Va) 1976;4:267–274. [PubMed] [Google Scholar]
- 2.Piersma A H, Ploemacher R E, Brockbank K G M. Br J Haematol. 1983;54:285–290. doi: 10.1111/j.1365-2141.1983.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 3.Mardon H J, Bee J, von der Mark K, Owen M E. Cell Tissue Res. 1987;250:157–165. doi: 10.1007/BF00214667. [DOI] [PubMed] [Google Scholar]
- 4.Owen M E, Friedenstein A J. Cell and Molecular Biology of Vertebrate Hard Tissues: Ciba Foundation Symposium, 136. New York: Wiley; 1988. pp. 42–60. [PubMed] [Google Scholar]
- 5.Beresford J N, Bennett J H, Devlin C, Leboy P S, Owen M E. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 6.Cheng S-L, Yang J W, Rifas L, Zhang S-F, Avioli L V. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 7.Caplan A I. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Rickard D J, Sullivan T A, Shenker B J, Leboy P S, Kaghdan I. Dev Biol. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 9.Wakitani S, Saito T, Caplan A I. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 10.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 11.Deryugina E I, Müller-Sieburg C E. Crit Rev Immunol. 1993;13:115–150. [PubMed] [Google Scholar]
- 12.Klein G. Experientia. 1995;51:914–926. doi: 10.1007/BF01921741. [DOI] [PubMed] [Google Scholar]
- 13.Simmons P J, Torok-Storb B. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 14.Long M W, Robinson J A, Ashcraft E A, Mann K G. J Clin Invest. 1995;95:881–887. doi: 10.1172/JCI117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller E K, Huang S, Terstappen L. Blood. 1995;86:710–718. [PubMed] [Google Scholar]
- 16.Gronthos S, Simmons P J. J Hematother. 1996;5:15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- 17.Bruder S P, Jaiswal N, Haynesworth S E. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Rickard D J, Kassem M, Hefferan T, Sarkar G, Spelsberg T C, Riggs B L. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 19.Friedenstein A J, Ivanov-Smolenski A A, Chajlakjan R K, Gorkaya U F, Kuralesova A I, Latzinik N W, Gerasimow U W. Exp Hematol (Charlottesville, Va) 1978;6:440–444. [PubMed] [Google Scholar]
- 20.Laver J, Jhanwar S C, O’Reilly R J, Castro-Malaspina H. Blood. 1987;70:1966–1968. [PubMed] [Google Scholar]
- 21.Simmons P J, Przepiorka D, Thomas E D, Torok-Storb B. Nature (London) 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 22.Athanasou N A, Quinn J, Brenner M K, Prentice H G, Graham A, Taylor S, Flannery D, McGee J O. Br J Cancer. 1990;61:385–389. doi: 10.1038/bjc.1990.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santucci M A, Trabetti E, Martinelli G, Buzzi M, Zaccaria A, Pileri S, Farabegoli P, Sabattini E, Tura S, Pignatti P F. Bone Marrow Transplant. 1992;10:255–259. [PubMed] [Google Scholar]
- 24.Tanaka J, Kasai M, Imamura M, Masauzi N, Ohizumi H, Matsuura A, Morii I, Kiyama Y, Naohara T, Saitoh M, et al. Br J Haematol. 1994;86:436–438. doi: 10.1111/j.1365-2141.1994.tb04764.x. [DOI] [PubMed] [Google Scholar]
- 25.Keating A, Singer J W, Killen P D, Striker G E, Salo A C, Sanders J, Thomas E D, Thorning D, Fialkow P J. Nature (London) 1982;298:280–283. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- 26.Marshall M J, Nisbet N W, Evans S. Experientia. 1984;40:385–386. doi: 10.1007/BF01952566. [DOI] [PubMed] [Google Scholar]
- 27.Anklesaria P, Kase K, Glowacki J, Holland C A, Sakakeeny M A, Wright J A, FitzGerald T J, Lee C Y, Greenberger J S. Proc Natl Acad Sci USA. 1987;84:7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zant G, Holland B P, Elbridge P W, Chen J J. J Exp Med. 1990;171:1547–1565. doi: 10.1084/jem.171.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D D, Keating A. Exp Hematol (Charlottesville, Va) 1991;19:485. (abstr). [Google Scholar]
- 30.Pereira R F, Halford K W, O’Hara M D, Leeper D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khillan J S, Olsen A S, Kontusaari S, Sokolov B, Prockop D J. J Biol Chem. 1991;266:23373–23379. [PubMed] [Google Scholar]
- 32.Sokolov B P, Mays P K, Khillan J S, Prockop D J. Biochemistry. 1993;32:9242–9249. doi: 10.1021/bi00086a033. [DOI] [PubMed] [Google Scholar]
- 33.Sokolov B P, Ala-Kokko L, Dhulipala R, Arita M, Khillan J S, Prockop D J. J Biol Chem. 1995;270:9622–9629. doi: 10.1074/jbc.270.16.9622. [DOI] [PubMed] [Google Scholar]
- 34.Pereira R, Khillan J S, Helminen H J, Hume E L, Prockop D J. J Clin Invest. 1993;91:709–716. doi: 10.1172/JCI116252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curland J I. In: Hematopoiesis. Golde D W, editor. New York: Churchill Livingston; 1984. pp. 95–100. [Google Scholar]
- 36.Woessner J F., Jr Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 37.Fiedler-Nagy C, Bruckner P, Hayashi T, Fietzek P P, Prockop D J. J Biol Chem. 1982;257:9181–9188. [Google Scholar]
- 38.Bishop C E, Hatat D. Nucleic Acids Res. 1987;15:2959–2968. doi: 10.1093/nar/15.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinkel D, Landegent J, Collins C, Fuscoe J, Segraves R, Lucas J, Gray J. Proc Natl Acad Sci USA. 1988;85:9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Constantinou C D, Pack M, Young S B, Prockop D J. Am J Hum Genet. 1990;47:670–679. [PMC free article] [PubMed] [Google Scholar]
- 41.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, Alt F W. Cell. 1992;68:855–857. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 41a.Chen J, Shinkai Y, Young F, Alt F W. Curr Opin Immunol. 1994;6:313–319. doi: 10.1016/0952-7915(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 42.Keating A, Guinn B, Larava P, Wang X-H. Exp Hematol (Charlottesville, Va) 1996;24:180. [Google Scholar]
- 43.Guinn B, Wang X-H, Larava P, Keating A. Blood. 1996;88S:3921. [Google Scholar]
- 44.Hou Z, Frenkel B, Lian J, Nilsson S, van Wijnen A J, Stein J, Quesenberry P, Stein G. J Bone Miner Res. 1997;12:5428. [Google Scholar]
- 45.Nilsson S K, Dooner M S, Weier H-U, Frenkel B, Lian J B, Stein G, Quesenberry P J. Blood. 1997;905:316b. [Google Scholar]
- 46.Onyia, J. E., Clapp, D. W., Vaughn, H. & Hock, J. M. (1997) J. Bone Miner. Res., in press. [DOI] [PubMed]
- 47.Nakagawa S, Toritsuka Y, Wakitani S, Denno K, Tomita T, Owaki H, Kimura T, Shino K, Ochi T. J Rheumatol. 1996;23:2098–2103. [PubMed] [Google Scholar]
- 48.Eglitis M A, Mezey E. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossert J A, Chen S S, Eberspaecher H, Smith C N, de Crombrugghe B. Proc Natl Acad Sci USA. 1996;93:1027–1031. doi: 10.1073/pnas.93.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark B R, Keating A. Ann N Y Acad Sci. 1995;770:70–78. doi: 10.1111/j.1749-6632.1995.tb31044.x. [DOI] [PubMed] [Google Scholar]