Abstract
Despite marked advances in the understanding of allergic responses, the mechanisms regulating gastrointestinal allergy are not very well understood. We have developed a model of antigen-induced eosinophil-associated gastrointestinal allergy and characterized the role of eotaxin and IL-5. Challenge of allergen-sensitized mice with oral allergen, in the form of enteric-coated beads, resulted in marked allergen-specific IgG1 and IgE, Th2-type (IL-4 and IL-5) cytokine production, and eosinophil accumulation in the blood and small intestine. In the genetic absence of eotaxin, a chemokine constitutively expressed in the gastrointestinal tract, eosinophil recruitment into the small intestine was ablated, and these mice developed enhanced eosinophil accumulation in the blood compared with wild-type mice. Interestingly, in the absence of IL-5, allergen challenge promoted partial eosinophil accumulation into the small intestine and a decline in circulating eosinophil levels. Collectively, these results establish that the accumulation of gastrointestinal eosinophils is antigen induced, can occur independent of IL-5, and provides a molecular mechanism to explain the dichotomy between peripheral blood and tissue eosinophilia. Furthermore, eotaxin is identified as a critical regulator of antigen-induced eosinophilic inflammation in the gastrointestinal tract.
Allergic diseases have reached epidemic proportions in the Western world, affecting nearly 30 percent of the population (1). Interestingly, this increased prevalence is paralleled by an increase in the severity and spectrum of disorders involving hypersensitivity responses in various tissues (e.g., the gastrointestinal tract). Although substantial progress has been made in elucidating the inflammatory mechanisms involved in allergic responses in the lung, there has been limited progress in understanding the pathogenesis of allergic disorders of the gastrointestinal tract. The development of experimental models of allergy has provided important insights into the immunological mechanisms regulating systemic (e.g., anaphylaxis) and pulmonary (e.g., asthma) allergic diseases. Collectively, these studies have identified a central role for cytokines (e.g., IL-4, IL-5, and IL-13), CD4+ T cells, mast cells, and, in particular, eosinophils, in the induction and sustainment of allergic inflammatory responses (2).
Eosinophil accumulation in the peripheral blood and tissues is a hallmark of allergic responses, and clinical investigations have established a strong link between the pathobiology of several allergic disorders and eosinophil accumulation and activation (3). However, it remains to be determined why some disease states are characterized by peripheral blood eosinophilia, whereas others are associated with a tissue eosinophilia in the presence or absence of peripheral blood eosinophilia. Although most studies have demonstrated an integral role for the cytokine IL-5 in eosinophil trafficking during allergic inflammatory responses, chemokines have also been recently implicated in the regulation of eosinophil accumulation (4–6). In particular, eotaxin has been identified as a potent and selective eosinophil chemoattractant and has been implicated in the pathogenesis of human allergic disease (7). However, in contrast to mice deficient in IL-5, aeroallergen challenge of eotaxin-deficient mice induces eosinophilic airway inflammation (8–10). Collectively, these data suggest that in comparison to eotaxin and other CCR3-ligands, IL-5 plays an obligatory role in regulating eosinophil trafficking during allergic responses in the lung.
There are a spectrum of eosinophil-associated inflammatory responses in the gastrointestinal tract, including IgE-mediated food anaphylaxis, inflammatory bowel disease, gastroesophageal reflux, allergic eosinophilic gastroenteritis, and eosinophilic colitis (11). It is currently thought that eosinophils may augment and sustain the gastrointestinal inflammatory response through the release of inflammatory mediators and/or granule cationic proteins that are toxic to the mucosa (11–14). However, although there have been recent advances in modeling some of these disease processes (e.g., IgE-mediated anaphylaxis responses), there have been only limited models of eosinophil-associated gastrointestinal allergy, and the precise mechanisms regulating gastrointestinal eosinophilia and the immunopathological role of this leukocyte in gastrointestinal disorders remain an enigma (11, 13, 15). To elucidate these processes, we have developed a murine model of eosinophil-associated gastrointestinal allergy and examined the role of eotaxin and IL-5 in the regulation of eosinophil trafficking.
Methods
Animals.
Eotaxin-deficient inbred mice of the (129/SvEv) strain were maintained with age, and sex-matched controls were obtained from Taconic Farms as previously described (9). IL-5-deficient inbred mice of the (BALB/c) strain and age and sex-matched controls were kindly provided by K. Matthaei (John Curtin School of Medical Research, Canberra, Australia) (8). Mice were sensitized by i.p. injection with 50 μg ovalbumin (OVA)/1 mg alum in 0.9% sterile saline on day 0. On days 12 and 15, mice were lightly anesthetized with Metofane inhalation (methoxy-fluorane; Pittman–Moore, Mundelein, IL) and orally administered 20 mg of encapsulated OVA enteric-coated beads or encapsulated placebo enteric-coated beads followed by oral administration of 300 μl of acidified H20 (pH 2.0) (16). Seventy-two hours after the last antigen challenge, mice were killed by cervical dislocation and parameters measured.
ELISA Measurements.
Serum OVA-specific IgG1 and IgE concentrations were determined by ELISA. Sample wells were coated with OVA (100 μg/ml) for IgG1 or anti-mouse IgE (EM-95; 10 μg/ml; gift from F. Finkelman, University of Cincinnati, Cincinnati, OH), blocked with 10% FBS in PBS, and washed with 0.05% Tween-20 in PBS. Serum samples were diluted 1:5 for IgE and 1:1,000 for IgG1 with 10% FCS in PBS and serially diluted (1:2). After a 2-h incubation at 37°C, plates were washed with 0.05% Tween-20 in PBS, and biotin-conjugated anti-mouse IgG1 (clone: A85–1; 0.5 μg/ml; PharMingen) or biotinylated OVA (4 μg/ml) was added. By using streptavidin horseradish peroxidase detection (2 μg/ml; ImmunoTech, Marseilles, France), the OD was read at 490 nm within 30 min. Data represent mean ± SEM of the serum dilution required to obtain an OD = 0.4. Values are representative of n = 4–5 mice per group from triplicate experiments.
Antigen-Specific T-Cell Response.
Splenocytes were subjected to OVA or αCD3/αCD28 stimulation as previously described (17). For proliferation experiments, splenocyte cultures were pulsed with 1 μCi [3H]-thymidine for the last 6 h of a 72-h culture. IL-4, IL-5, and IFNγ levels were determined in the supernatants from OVA- (50 μg/ml) or αCD3- (5 μg/ml)/αCD28 (1 μg/ml) stimulated splenocytes homogenates by using the OptEIA Mouse IL-4 and IL-5 kits (PharMingen), respectively. The IFNγ level was measured by using rat anti-mouse IFNγ mAb (PharMingen; 5 μg/ml, clone; R4–6A2). The sensitivity of the ELISA system was 20 pg/ml for IL-4 and IL-5 and 100 pg/ml for IFNγ.
Immunohistochemistry.
The small intestine of mice was divided into the duodenum, jejunum, and ileum, and segments of the gastrointestinal tract were fixed with paraformaldehyde, processed by using standard histological techniques, and immunostained with antiserum against mouse major basic protein (MBP) as previously described (18). Briefly, 5-μm sections were quenched with H202, blocked with normal goat serum, and stained with a rabbit antimurine eosinophil MBP antiserum (gift of J. and N. Lee, Mayo Clinic, Scottsdale, AZ). The slides were washed and incubated with biotinylated goat anti-rabbit antibody and avidin–peroxidase complex (Vectastain ABC Peroxidase Elite kit; Vector Laboratories). The slides were then developed by nickel diaminobenzidine, enhanced cobalt chloride to form a black precipitate, and counterstained with nuclear fast red. Quantification of immunoreactive cells was performed by morphometric analysis by using the Metamorph Imaging System (Universal Imaging, West Chester, PA). The sections were taken from the same position in the jejunum (3–5 cm distal to the stomach), and at least four to five random sections per mouse were analyzed. Values were determined by quantifying the total MBP+ pixel number relative to the total pixel counts of the lamina propria and mucosa regions of the gastrointestinal tissue. Eosinophil levels are expressed as the MBP staining/area (%).
FACS Analysis on Peripheral Blood Eosinophils.
Peripheral blood was subjected to red blood cell lysis, and cells (106) were incubated with 1 μg phycoerythrin-conjugated anti-mouse LPAM-1 (Integrin α4β7 complex; DATK32; PharMingen) and FITC-conjugated anti-mouse CCR3 mAb (1/200; DNAX) or the isotype-matched control antibodies at 4°C (19). Cells were analyzed on a FACScan Flow cytometer (Becton Dickinson) by using cellquest software (Becton Dickinson).
Results
Development of Experimental Antigen-Induced Eosinophilic Gastrointestinal Allergy.
One of the complexities of inducing allergic inflammation of the gastrointestinal tract is the ineffectiveness of orally administered soluble protein antigens in promoting hypersensitivity responses; rather, oral antigens generally promote immunological tolerance (20). The poor immune response and induction of oral tolerance is thought to be associated, at least in part, with gastric digestion of soluble protein antigens, which leads to the formation of nonimmunogenic peptides (21, 22). To overcome immunological tolerance associated with oral administration of soluble antigens (23, 24), we used a system whereby a soluble protein antigen was encapsulated to protect against gastric digestion (16). The encapsulated biodegradable antigen particles are resistant to degradation at gastric pH (pH 2.5); however, they are susceptible to degradation at pH 5.5, which facilitates the delivery and release of the allergen in a preserved native conformational state to the small intestine (16). Extensive previous investigations have demonstrated that the particles may overcome immunological tolerance associated with oral administration of antigens and possess adjuvant and immunostimulatory activity promoting antigen-specific antibody production (25).
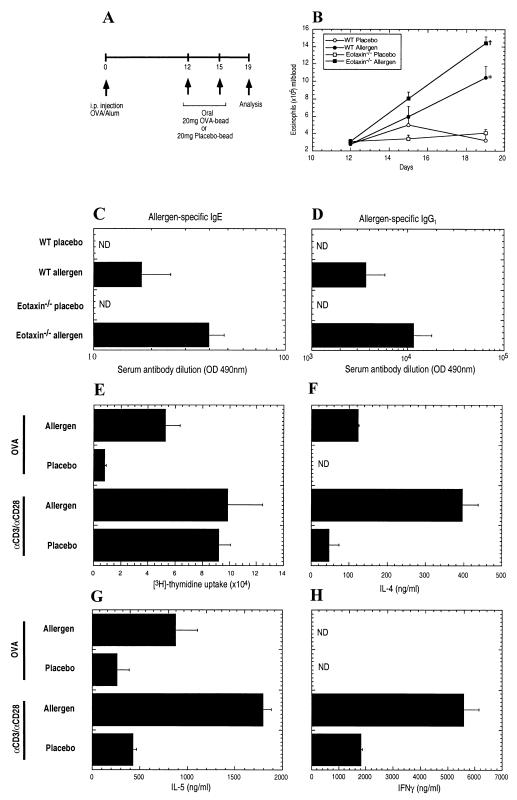
Mice were injected i.p. with the egg antigen OVA in the presence of adjuvant (alum) and subsequently challenged with oral OVA particles on days 12 and 15 (Fig. 1A). Administration of oral allergen to sensitized mice induced peripheral blood eosinophilia and allergen-specific IgE and IgG1, but not IgG2a, antibody responses (Fig. 1 B–D; data not shown). On day 19, the level of eosinophils in the peripheral blood of oral allergen-challenged mice was 3.5-fold higher than in placebo-challenged mice (Fig. 1B). To establish whether the peripheral blood eosinophilia and antigen-specific IgE was associated with the development of a CD4+ Th2-type immune response, proliferation and cytokine responses by splenic T cells from oral allergen- or placebo-challenged mice were determined (Fig. 1 E–H). Allergen stimulation induced a significant proliferation response in the oral allergen-challenged mice but not the control group (Fig. 1E). In contrast, the proliferation responses of T cells to nonspecific polyclonal activation (αCD3/αCD28) was comparable between placebo- and oral allergen-challenged mice (Fig. 1E). To characterize the phenotype of the allergen-reactive T cells, IL-4, IL-5, and IFNγ levels in the splenocyte cultures were measured (Fig. 1 F–H). The addition of OVA to the T cells of oral allergen-challenged mice induced the production of IL-4 and IL-5, but no detectable IFNγ, a cytokine produced by Th1 cells. In contrast, splenocytes isolated from placebo-challenged mice produced only low levels of IL-5 and no detectable IL-4 or IFNγ (Fig. 1 F–H). Collectively, these data indicated that oral allergen challenge promoted the expansion of a Th2-biased immune response.
Figure 1.
Characterization of gastrointestinal allergy in wild-type and eotaxin-deficient mice. (A) Experimental regime. Mice were i.p. injected with 50 μg OVA/1 mg alum on day 0. On days 12 and 15, mice were orally administered 20 mg of encapsulated placebo- and OVA-enteric coated beads followed by acidified H2O (pH 2.0). Four days after the last challenge, mice were killed and parameters measured. (B) Eosinophil numbers in the peripheral blood of placebo- and oral-allergen-challenged wild-type (WT) and eotaxin-deficient (eotaxin−/−) mice. Inset specifies the groups. (C and D) Levels of allergen-specific IgE (C) and IgG1 (D) in the serum of placebo- and oral allergen-challenged wild-type (WT) and eotaxin-deficient (eotaxin−/−) mice were determined by ELISA. (E) Proliferation of splenic T cells stimulated by αCD3/αCD28 or OVA were measured by [3H]-thymidine incorporation. (F–H) Secretion of IL-4 (F), IL-5 (G), and IFNγ (H) by αCD3/αCD28- and OVA-stimulated splenic T cells. Data in B and D represent the mean ± SEM for groups of four to five animals in triplicate experiments and (E–H) mean ± SEM from three individual cultures obtained from four to five mice in each group. Statistical significance of experimental groups was analyzed by using the unpaired Student's t test (B). *, P < 0.05 compared with day 19 allergen challenge of wild-type mice; +, P < 0.001 compared with placebo challenge of wild-type mice on day 19. Unless otherwise indicated, data were obtained on day 19. ND, not detected.
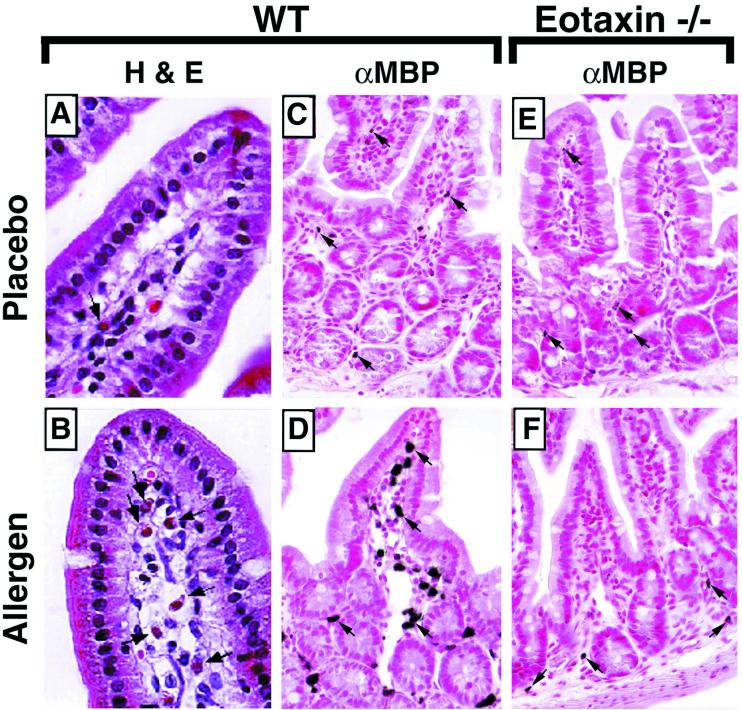
Histological examination of the jejunum revealed vascular congestion, edema, and a prominent cellular infiltrate in the oral allergen-challenged mice as compared with placebo-challenged mice (Fig. 2 A and B). The cellular infiltrate was predominantly localized to the mucosa and lamina propria throughout the small intestine and was primarily composed of eosinophils (Fig. 2B, and results not shown). Elevated levels of eosinophils were also observed in the duodenum and ileum of oral allergen-challenged mice as compared with placebo-challenged mice (results not shown). The presence of eosinophils was further characterized by standard immunohistochemical analysis by using an antiserum specific for eosinophils (anti-MBP) (Fig. 2 C and D). The infiltrating eosinophils were observed interspersed throughout the reticular connective tissue of the lamina propria and mucosa and throughout the length of the lamina propria of the villi (Fig. 2 B and D). Placebo-challenged mice had low levels of eosinophils predominantly localized to the base of the villus in the region of the crypt of Lieberkühn and occasional cells within the lamina propria of the villus (Fig. 2 A and C). This distribution is similar to the location of eosinophils at baseline (naïve mice), indicating that placebo challenge alone did not significantly affect eosinophil trafficking (18). Morphometric analysis revealed that the level of eosinophils in oral allergen-challenged mice was significantly higher (P < 0.0001) than that observed in placebo-challenged mice (Fig. 3). Because mast cells have also been implicated in the pathogenesis of various gastrointestinal hypersensitivity responses (11, 13), we were interested in examining their participation in oral antigen-induced eosinophil gastrointestinal allergy. Histological analysis of the jejenum revealed no significant difference in the level of mast cells within the lamina propria and villus between placebo-challenged and oral allergen-challenged mice (results not shown).
Figure 2.
Histological analysis of jejunum tissue from placebo- and oral allergen-challenged wild-type and eotaxin-deficient mice. (A and B) Photomicrographs represent hemataoxylin- and eosin-stained jejunum sections from placebo- (A) and oral allergen- (B) challenged wild-type mice. (C and D) Photomicrographs represent rabbit anti-mouse MBP [αMBP]-immunostained jejunum sections from placebo- (C) and oral allergen- (D) challenged wild-type mice. (E and F) Photomicrographs represent anti-MBP-stained jejunum sections from placebo- (E) and oral allergen- (F) challenged eotaxin-deficient mice. In placebo-challenged wild-type mice (C), eosinophils are predominantly localized to the crypt region and less frequently within the lamina propria of the villus. In oral allergen-challenged wild-type mice (B and D) but not eotaxin-deficient mice (F), an eosinophilic infiltrate is observed. Eosinophils are present within the mucosa and throughout the length of the villus. Arrows depict representative eosinophils. (A and B, ×950; C–F, ×460.)
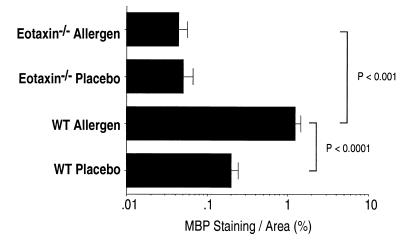
Figure 3.
Quantification of gastrointestinal eosinophils by morphometric analysis in placebo- and oral allergen-challenged wild-type and eotaxin-deficient mice. Gastrointestinal eosinophil numbers were quantified in placebo- and oral allergen-challenged wild-type (WT) and eotaxin-deficient (eotaxin−/−) mice by morphometric analysis. Data were obtained on day 19. Data represent the mean ± SEM of four to five random sections/mouse for four to five animals per group and are representative of three separate experiments. Statistical significance of experimental groups was analyzed by using the unpaired Student's t test.
Critical Role of Eotaxin in Antigen-Induced Experimental Eosinophilic Gastrointestinal Allergy.
Elevated levels of eotaxin and eosinophils have been associated with various human inflammatory disorders, and increased levels correlate with disease severity (26, 27). Thus, it was critical to determine the role of this chemokine and gastrointestinal eosinophils in gastrointestinal allergic inflammation. Oral allergen challenge of eotaxin-deficient mice induced a marked increase in peripheral blood eosinophils compared with placebo-challenged eotaxin-deficient mice (Fig. 1B). Interestingly, the peripheral blood eosinophilia was significantly higher than that of oral allergen-challenged wild-type mice (Fig. 1B). We hypothesized that the elevated level of eosinophils in the peripheral blood of oral allergen-challenged eotaxin-deficient mice was because of failure to recruit eosinophils to the gastrointestinal tract, thus preventing the transmigration of circulating eosinophils into the site of inflammation. To test this hypothesis, we examined the level of eosinophils in the lamina propria of the small intestine of oral allergen-challenged eotaxin-deficient mice. Histological analysis of the intestinal tissue from oral allergen-challenged mice revealed no significant morphological changes to the small intestine structural integrity in the absence of eotaxin (Fig. 2F; data not shown). Morphometric analysis of anti-MBP-stained tissue revealed that in contrast to wild-type mice, oral allergen challenge of eotaxin-deficient animals induced no significant increase in the level of eosinophils as compared with placebo-challenged eotaxin-deficient mice (Fig. 3). The level of eosinophils in oral allergen-challenged eotaxin-deficient mice was markedly reduced compared with wild-type mice (P < 0.001) (Fig. 3). This indicates that the reduced baseline level of gastrointestinal lamina propria eosinophils in eotaxin-deficient mice is not increased by allergen challenge (28). The reduction of intestinal inflammation in the absence of eotaxin was not because of the failure to develop allergen-specific lymphocyte responses, because eotaxin-deficient mice produced marked levels of allergen-specific IgE and IgG1 (Fig. 1 C and D) and Th2 cytokines (data not shown).
Normal Levels of CCR-3 and α4β7 in the Absence of Eotaxin.
We next examined the mechanism for the impaired recruitment of eosinophils into the gastrointestinal tract in the absence of eotaxin. Recent investigations have demonstrated that other chemokines, including RANTES, may induce eosinophil chemotaxis via the eotaxin receptor, CC-chemokine receptor 3 (CCR3) (6, 29, 30). To determine whether ablation of eosinophil transmigration was specific for eotaxin and was not because of a loss of CCR3, we examined the level of expression of CCR3 on peripheral blood eosinophils from oral allergen-challenged wild-type and eotaxin-deficient mice. We observed no difference in the level of CCR3 expression between wild-type and eotaxin-deficient mice, suggesting that eotaxin was not required for CCR3 expression [the mean fluorescence intensity for CCR3 was 23.8 and 21.8 for eosinophils from wild-type and eotaxin-deficient mice, respectively; see supplementary data (www.pnas.org)]. We also examined the level of expression of the eosinophil adhesion molecule, α4β7, because this integrin has been shown to be responsible for the recruitment of leukocytes into the intestinal lamina propria and associated lymphatic organs (31, 32). Eosinophils from the peripheral blood of oral allergen-challenged wild-type mice expressed α4β7 at the same level as eosinophils from eotaxin-deficient allergen-challenged mice (the mean fluorescence intensity for α4β7 was 8.56 and 9.82 for eosinophils from wild-type and eotaxin-deficient mice, respectively; see supplementary data). Collectively, these results suggest that the failure to recruit eosinophils into the intestine of allergen-challenged mice is most likely because of loss of the eotaxin concentration gradient in the intestine.
The Role of IL-5 in Experimental Eosinophilic Gastrointestinal Allergy.
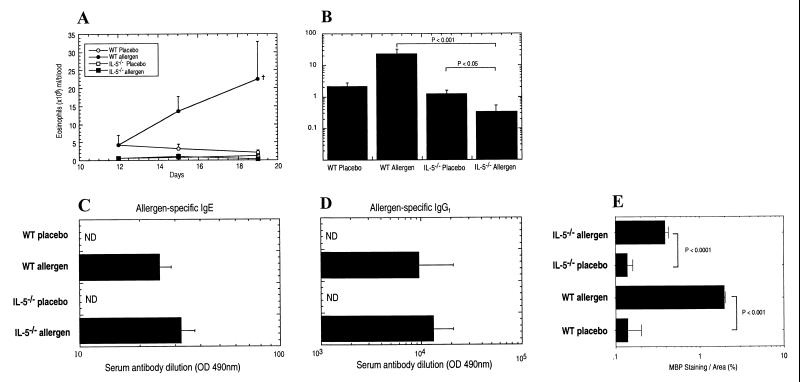
We were next interested in determining the role of IL-5 in regulating eosinophil-associated gastrointestinal allergy because this cytokine is a pivotal modulator of eosinophil trafficking during allergic airways inflammation (8, 17, 33, 34). IL-5 has been shown to mobilize eosinophils from the bone marrow into the circulation and to promote eosinophil tissue trafficking during allergic airway inflammation (8, 35, 36). We therefore compared oral allergen-induced gastrointestinal allergy in IL-5-deficient and wild-type mice. In marked contrast to wild-type mice, allergen challenge of IL-5-deficient mice did not promote a peripheral blood eosinophilia (Fig. 4 A and B). Interestingly, after the second allergen challenge (day 19), the level of eosinophils in the blood of these mice was significantly lower than that of placebo-challenged IL-5-deficient mice (P < 0.05; Fig. 4B). Levels of allergen-specific IgE and IgG1 resembled those present in wild-type mice (Fig. 4 C and D). These data suggest that the limited number of circulating eosinophils present in IL-5-deficient animals were being recruited into the intestine after allergen challenge, thereby depleting the level of peripheral blood eosinophils. To prove this, morphometric analysis of anti-MBP staining of IL-5-deficient mice revealed a 3-fold increase in the level of eosinophils recruited into the intestine after allergen challenge (Fig. 4E). The level of eosinophil recruitment was still lower than in allergen-challenged wild-type mice. These studies also indicated that the baseline production of eosinophils, which occurs independently of known eosinophil hematopoietins (IL-3, IL-5, and GM-CSF) (18), provides a sufficient number of eosinophils for the development of tissue eosinophilia.
Figure 4.
Gastrointestinal allergy in IL-5-deficient mice. (A) Eosinophil numbers in the peripheral blood of placebo- and allergen-challenged wild-type and IL-5-deficient mice. Eosinophil numbers in the peripheral blood of placebo- and allergen-challenged wild-type and IL-5-deficient mice on days 12, 15, and 19 are shown. (B) Eosinophil numbers in the peripheral blood of placebo- and allergen-challenged wild-type and IL-5-deficient mice on day 19. (C and D) The level of allergen-specific IgE (C) and IgG1 (D) in serum of placebo- and allergen-challenged wild-type and IL-5-deficient mice. (E) Eosinophil numbers in the jejunum were quantified by morphometric analysis. Data represent the mean ± SEM for (A–E) SEM of four to five random sections/mouse for four to five animals per group and are representative of two separate experiments. Statistical significance of experimental groups was analyzed by using the unpaired Student's t test (A). +, P < 0.01 compared with placebo-challenged wild-type (WT) mice.
Discussion
We have developed a model for oral allergen-induced eosinophil-associated gastrointestinal allergy that mimics a variety of human gastrointestinal allergic conditions. Although no murine model adequately mimics human disease, our experimental regime offers an experimental framework to analyze the events associated with antigen-induced eosinophil-associated gastrointestinal allergy. Our results establish that oral allergen is sufficient to promote the recruitment of eosinophils to the gastrointestinal tract. Interestingly, it was previously known that the level of gastrointestinal eosinophils in a subset of patients with eosinophilic gastrointestinal inflammation decreases after a specific food elimination diet, but no causal link between allergen sensitization and exposure and gastrointestinal eosinophil levels was established (11, 13). Importantly, we have identified eotaxin as a key regulator of eosinophil trafficking during gastrointestinal allergic processes. It has previously been shown that eotaxin has a nonobligatory role in regulating allergic responses in the lung because eosinophil recruitment and airway hyperreactivity during the sustained late phase response developed in allergen-challenged eotaxin-deficient mice (9, 10). Furthermore, antibody neutralization studies with bronchoalveolar lavage fluid from humans with asthma have suggested only a partial role for eotaxin in the pathogenesis of asthma (26). In the present study, allergen-induced eosinophilic infiltration in the gastrointestinal tract was shown to be markedly impaired in the absence of eotaxin, suggesting that the mechanism for eosinophil homing into this mucosal tissue differs from that operational in the lung (9, 10).
Only a limited number of experimental systems have been reported to mimic eosinophilic gastrointestinal diseases. For example, in one system, anaphylaxis was induced in mice that were injected with hybridoma cells secreting anti-TNP IgE (37). These experimental systems, although reporting eosinophilic inflammation, have not examined the mechanisms involved in eosinophil trafficking or effector function. Several other gastrointestinal allergy models have been reported, but these mimic noneosinophilic gastrointestinal allergic disorders. For example, allergen treatment of mice passively sensitized with IgE results in mast cell-dependent neutrophil recruitment (38). Furthermore, sensitization with allergen and cholera toxin induced mast cell degranulation and anaphylaxis but no eosinophil recruitment into the gastrointestinal tract (39).
Our data also provide an explanation for the dichotomy that is often observed between peripheral blood and tissue eosinophilia in various diseases (40). For example, patients with gastroesophageal reflux have eosinophilia in the esophagus but rarely have elevated circulating eosinophil numbers, and only a subset of patients with asthma has peripheral blood eosinophilia. Furthermore, drug-induced eosinophilia is usually limited to the peripheral blood (40). The finding that peripheral blood and tissue eosinophilia can be dissociated in the absence of eotaxin indicates that the relative balance between the expression of eotaxin and eosinophil hematopoietins (e.g., IL-5) can have profound effects on the relative distribution of eosinophils. As a corollary, underexpression of IL-5 relative to eotaxin can lead to gastrointestinal tissue eosinophilia in the absence of circulating eosinophilia. This is supported by the finding that oral allergen challenge of mice deficient in IL-5 results in a decrease in the circulating level of eosinophils compared with wild-type mice.
In summary, this investigation provides insight into the molecular mechanisms involved in allergic responses of the gastrointestinal tract. We demonstrate that oral allergen challenge to sensitized mice promotes allergic inflammation characterized by Th2-associated eosinophil accumulation in the peripheral blood and small intestine. Additionally, we demonstrate the critical role of eotaxin in regulating allergen-induced eosinophil trafficking to the lamina propria of the gastrointestinal tract. Furthermore, we demonstrate that eosinophil recruitment to the gastrointestinal tract can occur in the absence of the major eosinophil growth factor IL-5. Lastly, we provide a molecular explanation to explain the dichotomy seen between peripheral blood and tissue eosinophilia in a variety of medical diseases. These data indicate that agents that block eotaxin and/or CCR3 may have beneficial effects on modulating eosinophil-associated gastrointestinal allergies. It is hopeful that this study will provide the necessary framework to examine allergic responses in the gastrointestinal tract with the same scrutiny that has been applied to the study of allergic processes in the lung.
Supplementary Material
Acknowledgments
We thank Connie Lobas for excellent technical assistance and F. Finkelman, M. Cohen, A. Srikiatkhachorn, G. K. Hershey, S. Wert, N. Zimmermann, and L. Poulos for critical reading of the manuscript and helpful discussions. We thank A. Lippelman for secretarial assistance and Alicia Emly for graphic assistance. We also thank Robert Coffman for the anti-CCR3 serum and James and Nancy Lee for anti-MBP serum. This work was supported in part by the National Health Medical Research Council (Australia), C. J. Martin Postdoctoral Fellowship (S.P.H.), the Jaffe Family Fund of the American Academy of Asthma, Allergy, and Immunology (S.P.H.), National Institutes of Health grant R01 AI45898–01 (M.E.R.), and the Human Frontier Science Program (M.E.R. and P.S.F.).
Abbreviations
- MBP
major basic protein
- OVA
ovalbumin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Holgate S T. Nature (London) 1999;402:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 2.Drazen J M, Arm J P, Austen K F. J Exp Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleich G J, Adolphson C R. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 4.Rollins B J. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 5.Luster A D. N Eng J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 6.Nickel R, Beck L A, Stellato C, Schleimer R P. J Allergy Clin Immunol. 1999;104:723–742. doi: 10.1016/s0091-6749(99)70281-2. [DOI] [PubMed] [Google Scholar]
- 7.Jose P J, Griffiths-Johnson D A, Collins P D, Walsh D T, Moqbel R, Totty N F, Truong O, Hsuan J J, Williams T J. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothenberg M E, MacLean J A, Pearlman E, Luster A D, Leder P. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Loy J, Ryseck R P, Carrasco D, Bravo R. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- 11.Sampson H A. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak A M, Onderdonk A B, McLeod R S, Monahan-Earley R A, Antonioli D A, Cullen J, Blair J E, Cisneros R, Letourneau L, Morgan E, et al. Int Arch Allergy Immunol. 1993;102:33–45. doi: 10.1159/000236548. [DOI] [PubMed] [Google Scholar]
- 13.Furuta G T, Ackerman S J, Wershil B K. Curr Opin Gastroenterol. 1995;11:541–547. [Google Scholar]
- 14.Kato M, Kephart G M, Talley N J, Wagner J M, Sarr M G, Bonno M, McGovern T W, Gleich G J. Anat Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelly K J. J Pediatr Gastroenterol Nutr. 2000;30:S28–S35. doi: 10.1097/00005176-200001001-00005. [DOI] [PubMed] [Google Scholar]
- 16.Litwin A, Flanagan M, Michael J G. Biodrugs. 1998;9:261–270. doi: 10.2165/00063030-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Hogan S P, Koskinen A, Matthaei K I, Young I G, Foster P S. Am J Respir Crit Care Med. 1998;157:210–218. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A, Hogan S P, Lee J J, Foster P S, Rothenberg M E. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimaldi J C, Yu N X, Grunig G, Seymour B W, Cottrez F, Robinson D S, Hosken N, Ferlin W G, Wu X, Soto H, et al. J Leukocyte Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- 20.Miller A, Lider O, al-Sabbagh A, Weiner H L. J Neuroimmunol. 1992;39:243–250. doi: 10.1016/0165-5728(92)90258-m. [DOI] [PubMed] [Google Scholar]
- 21.Mestecky J, McGhee J R, Arnold R R, Michalek S M, Prince S J, Babb J L. J Clin Invest. 1978;61:731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael J G. Immunol Invest. 1989;18:1049–1054. doi: 10.3109/08820138909030606. [DOI] [PubMed] [Google Scholar]
- 23.Mayer L, So L P, Yio X Y, Small G. Ann N Y Acad Sci. 1996;778:28–35. doi: 10.1111/j.1749-6632.1996.tb21111.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiner H L, Mayer L F. Ann N Y Acad Sci. 1996;778:xiii–xviii. doi: 10.1111/j.1749-6632.1996.tb21109.x. [DOI] [PubMed] [Google Scholar]
- 25.Challacombe S J, Rahman D, O'Hagan D T. Vaccine. 1997;15:169–175. doi: 10.1016/s0264-410x(96)00159-4. [DOI] [PubMed] [Google Scholar]
- 26.Lamkhioued B, Renzi P M, Abi-Younes S, Garcia-Zepeda E A, Allakhverdi Z, Ghaffar O, Rothenberg M E, Luster A D, Hamid Q. J Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- 27.Ying S, Meng Q, Zeibecoglou K, Robinson D S, Macfarlane A, Humbert M, Kay A B. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 28.Matthews A N, Friend D S, Zimmermann N, Sarafi M N, Luster A D, Pearlman E, Wert S E, Rothenberg M E. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalo J A, Lloyd C M, Wen D, Albar J P, Wells T N, Proudfoot A, Martinez A C, Dorf M, Bjerke T, Coyle A J, et al. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukacs N W, Oliveira S H, Hogaboam C M. J Clin Invest. 1999;104:995–999. doi: 10.1172/JCI8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamann A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 32.Steeber D A, Tang M L, Zhang X Q, Muller W, Wagner N, Tedder T F. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 33.Nakajima H, Iwamoto I, Tomoe S, Matsumura R, Tomioka H, Takatsu K, Yoshida S. Am Rev Respir Dis. 1992;146:374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 34.Hamelmann E, Oshiba A, Loader J, Larsen G L, Gleich G, Lee J, Gelfand E W. Am J Respir Crit Care Med. 1997;155:819–825. doi: 10.1164/ajrccm.155.3.9117011. [DOI] [PubMed] [Google Scholar]
- 35.Mould A W, Matthaei K I, Young I G, Foster P S. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palframan R T, Collins P D, Williams T J, Rankin S M. Blood. 1998;91:2240–2248. [PubMed] [Google Scholar]
- 37.Ohtsuka Y, Suzuki R, Nagata S, Oguchi S, Shimizu T, Yamashiro Y, Okumura K, Ra C. Pediatr Res. 1998;44:791–797. doi: 10.1203/00006450-199811000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Furuta G T, Schmidt-Choudhury A, Wang M Y, Wang Z S, Lu L, Furlano R I, Wershil B K. Gastroenterology. 1997;113:1560–1569. doi: 10.1053/gast.1997.v113.pm9352858. [DOI] [PubMed] [Google Scholar]
- 39.Li X M, Schofield B H, Huang C K, Kleiner G I, Sampson H A. J Allergy Clin Immunol. 1999;103:206–214. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 40.Rothenberg M E. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.