Abstract
The Belgrade (b) rat has an autosomal recessively inherited, microcytic, hypochromic anemia associated with abnormal reticulocyte iron uptake and gastrointestinal iron absorption. The b reticulocyte defect appears to be failure of iron transport out of endosomes within the transferrin cycle. Aspects of this phenotype are similar to those reported for the microcytic anemia (mk) mutation in the mouse. Recently, mk has been attributed to a missense mutation in the gene encoding the putative iron transporter protein Nramp2. To investigate the possibility that Nramp2 was also mutated in the b rat, we established linkage of the phenotype to the centromeric portion of rat chromosome 7. This region exhibits synteny to the chromosomal location of Nramp2 in the mouse. A polymorphism within the rat Nramp2 gene cosegregated with the b phenotype. A glycine-to-arginine missense mutation (G185R) was present in the b Nramp2 gene, but not in the normal allele. Strikingly, this amino acid alteration is the same as that seen in the mk mouse. Functional studies of the protein encoded by the b allele of rat Nramp2 demonstrated that the mutation disrupted iron transport. These results confirm the hypothesis that Nramp2 is the protein defective in the Belgrade rat and raise the possibility that the phenotype shared by mk and b animals is unique to the G185R mutation. Furthermore, the phenotypic characteristics of these animals indicate that Nramp2 is essential both for normal intestinal iron absorption and for transport of iron out of the transferrin cycle endosome.
Most, if not all, mammalian cells are capable of taking up iron by receptor-mediated endocytosis of diferric transferrin (Tf) bound to the transferrin receptor (TfR). Following internalization and acidification of the endosome, Tf and the TfR are recycled to the extracellular space and cell surface, respectively. Although the fates of these proteins are well understood, the path that iron takes to other intracellular sites is not. In particular, it is not known how iron is transported across the endosomal membrane.
Several inbred strains of rodents have functional defects in iron uptake and transport that define obligate intracellular steps in the iron metabolic pathway (1). Among these animals, the Belgrade (b) laboratory rat has an autosomal recessively inherited, hypochromic, microcytic anemia associated with defective cellular iron uptake (2–6). Dual-labeling studies have shown that although diferric Tf is taken up into b reticulocytes, the iron is poorly retained, and much is inappropriately recycled to the extracellular space along with Tf (6). Furthermore, this reticulocyte transport defect is also apparent when iron is presented in a non-Tf bound form, indicating that the functional deficit is not simply due to a failure to dissociate iron from Tf (7, 8). In addition to the erythroid abnormality, in vivo and in vitro studies have shown that b rats also have impaired intestinal absorption of iron as well as diminished acquisition of iron by other cell types (refs. 9 and 10; M.D.G., unpublished data). Overall, the functional abnormalities suggest that there is a defect in a membrane carrier of iron that is common to many tissues, including the erythron and the intestine.
Similar to the Belgrade rat, microcytic anemia (mk) mice have a severe, hypochromic, microcytic anemia that is associated with defects in erythroid iron utilization and intestinal iron uptake (11–14). Using a positional cloning approach to identify the gene defective in these mice, we identified a missense mutation in the transmembrane protein Nramp2 (15). Recent studies have shown that Nramp2 stimulates iron uptake in transient transfection assays and in Xenopus oocyte injection studies (M.A.S., unpublished data, and ref. 17). The activity of the mk allele is severely diminished, confirming both the function of Nramp2 and the identity of the mk mutation (M.A.S., unpublished data). Given the phenotypic similarities between the b rat and the mk mouse, we considered the possibility that Nramp2 might also be mutated in the b rat. We report the localization of the b gene to the proximal portion of rat chromosome 7, in a region having syntenic homology to the telomeric portion of mouse chromosome 15 where mouse Nramp2 and the mk phenotype were previously mapped. Characterization of the b allele of Nramp2 revealed a missense mutation that results in a glycine-to-arginine substitution at amino acid codon 185 (G185R). Surprisingly, this change is identical to the mutation identified in mk mice. The b allele encodes a protein with little or no activity in iron uptake assays. These findings further support a role for Nramp2 in cellular iron metabolism, particularly within the transferrin cycle endosome.
METHODS
Animals.
Anemic Belgrade rats were descendants of the original Belgrade colony, obtained from K. Kellar (Centers for Disease Control and Prevention, Atlanta, GA), who backcrossed them (10 generations) onto a Harlan Sprague–Dawley Wistar background. The animals were subsequently maintained as a closed colony in Buffalo, NY, by breeding homozygous affected (b/b) males to obligate heterozygous (+/b) females. Homozygous wild-type (+/+) animals were obtained by breeding heterozygotes and testing their progeny with homozygous affected animals for passage of the b allele. For linkage experiments, male b/b rats were crossed to Harlan Sprague–Dawley Fischer 344 (F344) females to generate heterozygous F1 females, which were backcrossed to their b/b male parent. Phenotypes of backcross animals were determined at 7–10 weeks of age by the evaluation of peripheral blood smears for hypochromia, microcytosis, and polychromatophilia. Interpretations of the smears were confirmed by microhematocrit and hemoglobin determinations and a red cell count on a Coulter Zf counter. Tail DNA samples were prepared with a QIAmp Tissue Kit (Qiagen).
Genotyping and Linkage Analysis.
DNA samples of parental and F1 animals were used to select a set of markers mapping to the centromeric portion of rat chromosome 7 that were polymorphic between F344 and the b strain. Existing markers for rat genes that defined a region of synteny between mouse chromosome 15 and rat chromosome 7, including peripheral benzodiazopine receptor (Bzrp, D7Mit12), preproacrosin (Acr, D7Mgh2), and peripherin (Per, D7Mit9), were non-polymorphic in this strain set and consequently could not be used in the backcross analysis. Ninety-two backcross animals were genotyped with the markers D7Mit1, D7Mit16 (Cypiid4), and D7Mit3, which were obtained from Research Genetics and assayed by using standard PCR conditions, polyacrylamide/urea gel electrophoresis, and [32P]radioactive detection. To develop a marker for rat Nramp2, primer pairs spanning the entire mouse Nramp2 cDNA coding sequence at approximately 240 bp intervals were used to amplify F344 and b/b rat genomic DNA. The mouse and rat Nramp2 genes are highly homologous on a nucleotide level, and most mouse primers amplified the cloned rat cDNA. Of eight primer pairs tested on genomic DNA, one (5′-TATCCCAAGGTCCCACGGAT-3′; 5′-GAGGGCCATGATAGTGATGA-3′) amplified Nramp2 transmembrane domain 4 (TM4) and was polymorphic, yielding products of approximately 890 and 940 bp in F344 and b/b samples, respectively, when fractionated on 1% agarose gels. Evaluation of linkage between the marker set and the b phenotype was performed with mapmanager (16).
Mutation Analysis.
Multiple overlapping partial cDNA clones of Nramp2 from the b and + alleles segregating in the Belgrade colony were obtained by subcloning reverse transcription (RT)-PCR products. Five micrograms of total RNA extracted from kidney with RNA STAT-60 (Leedo Medical Laboratories, Houston) was used in first-strand synthesis reactions primed with either random hexamers or oligo(dT). Oligonucleotide primers homologous to the mouse Nramp2 cDNA and either Taq (Boehringer Mannheim) or Pfu (Stratagene) thermostable DNA polymerase were used to amplify Nramp2 products that were subcloned into pCR2.1 (Invitrogen). Genomic TM4 PCR products from +/+ and b/b rats were also subcloned into pCR2.1. DNA sequencing reactions were performed with Sequenase (United States Biochemical/Amersham) by using standard dideoxy chain termination conditions according to the manufacturer’s instructions. All potential coding sequence polymorphisms were confirmed by sequencing both DNA strands and additional clones derived from separate PCRs.
Expression Constructs.
A full-length rat Nramp2 cDNA clone was isolated from a rat cerebellum library (provided by E. Neufeld, Boston) by using a probe amplified from the cloned mouse cDNA using the TM4 primers specified above. The 1,704-bp/568-aa ORF was amplified from this clone with Pfu polymerase and subcloned into the EcoRI site of pMT2 (primers: 5′-CCTCAGAATTCTACCATGGTGTTGGATCC-3′; 5′-GAGAAAGAATTCTTAAAGTCTTCTGGGC-3′). A mutant form, corresponding to the G185R mutation found in b rats, was constructed by using the QuikChange site-directed mutagenesis kit (Stratagene) and the allele-specific oligonucleotides, 5′-GGGTTCCCCTGTATGGTAGAGTCCTCATCACCATCGC-3′ and 5′-GCGATGGTGATGAGGACTCTACCATACAGGGGAACCC-3′ (mutation underlined).
Cell Culture and Transfection.
Human embryonic kidney (HEK293T) cells were grown in high-glucose DMEM (GIBCO/BRL) supplemented with 10% fetal calf serum (BioWhittaker), 1% penicillin/streptomycin, and 1% l-glutamine. For transient transfection assays, cells were seeded at a concentration of 5 × 106 cells per 90-mm plate. Eighteen hours after seeding, 5 μg of the pMT2-Nramp2 expression plasmid was transfected by using calcium phosphate precipitation.
Iron Uptake Assay.
Iron uptake was measured 48 hr after transfection. To make 55Fe-nitrilotriacetic acid (55Fe-NTA), 55FeCl3 (New England Nuclear) was added to 100 mM NTA in a 1:50 molar ratio. Transfected cells were washed twice with PBS prewarmed to 37°C, prior to incubating in 10 ml of prewarmed incubation buffer (25 mM Tris/25 mM Mes/140 mM NaCl/5.4 mM KCl/5 mM glucose/1.8 mM CaCl2/800 μM MgSO4/50 μM ascorbic acid, pH 6.0) containing 55Fe-NTA (1 μM 55Fe) at 37°C under 5% CO2 for 20 min. Ascorbic acid was used to promote the formation and maintenance of ferrous (Fe2+) iron. Using the Fe2+-specific indicator ferrozine (Sigma), iron in the incubation medium was determined to be predominantly in the Fe2+ state. To terminate the reaction, 30 ml of ice-cold PBS was added to the plate and aspirated into a 50-ml tube and placed on ice. The remaining adherent cells were removed by a 30-sec room temperature digestion with 1 ml of 0.25% trypsin-EDTA (GIBCO/BRL) and pooled with the aspirated incubation medium in PBS on ice. Cells were pelleted and washed two more times in 20 ml ice-cold PBS. To determine 55Fe uptake, the cell pellet was resuspended in 1 ml distilled water and radioactivity was measured in a Beckman LS3801 scintillation counter by using a band width of 0 to 350.
RESULTS
The Belgrade Phenotype and Rat Nramp2 Both Map to Rat Chromosome 7.
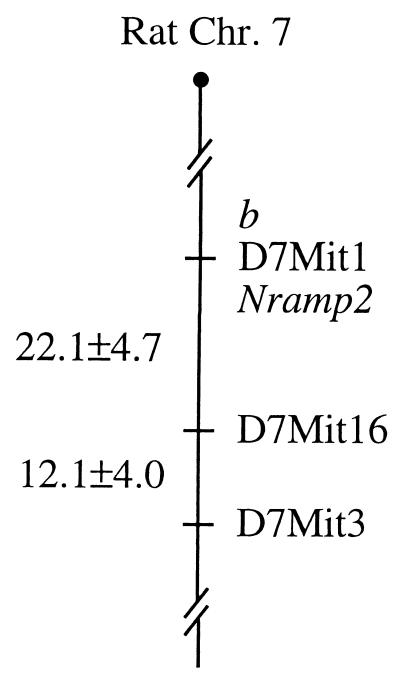
To evaluate the possibility that the b phenotype was due to a mutation in Nramp2, we first sought to determine whether b mapped to a region exhibiting syntenic homology to the distal portion of mouse chromosome 15 where mk had been mapped. Several genes mapping to this region of the mouse genome, including Bzrp, Acr, and Prph1, were located on the proximal portion of rat chromosome 7. The homologous interval appeared to be oriented such that the telomeric portion of mouse chromosome 15 was located near the centromere of chromosome 7 in the rat. Using a panel of 92 informative meioses from a Belgrade × F344 backcross, we constructed a map of the proximal portion of rat chromosome 7 and found that the b phenotype was nonrecombinant with the marker D7Mit1 (lod = 25.6, Fig. 1). This set of animals was also typed with a primer pair developed from the mouse Nramp2 cDNA, which amplified rat sequences and exhibited an intron-length polymorphism between b and F344 animals. No recombinations were identified between the Nramp2 marker and b or D7Mit1. The placement of b and rat Nramp2 on the proximal portion of this interval is comparable to the location of mk and Nramp2 in the mouse (15) and suggested that Nramp2 was a strong candidate gene for the b mutation.
Figure 1.
Nramp2 and the b phenotype map to proximal rat chromosome 7. Ninety-two [Belgrade × Fischer 344] F1 × Belgrade backcross animals were genotyped with microsatellite markers mapping to the centromeric portion of rat chromosome 7, and with a marker of rat Nramp2. The b phenotype was recombinationally inseparable from D7Mit1 and Nramp2 (lod = 25.6). Genetic distances and standard deviations, expressed in centiMorgans, are indicated on the left side of the figure, and markers are identified on the right.
Belgrade Rats Have a Glycine-to-Arginine Missense Mutation at Codon 185 of Nramp2.
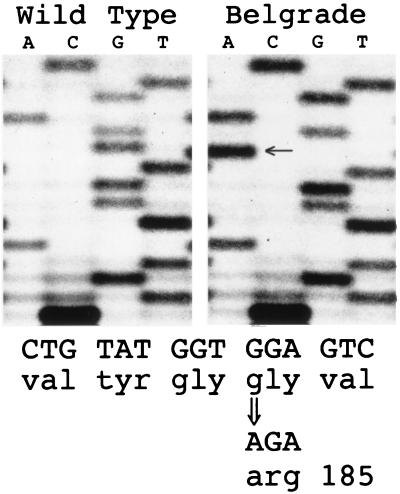
Primers homologous to the mouse and rat Nramp2 genes were used to obtain multiple overlapping partial RT-PCR cDNA clones of the b and wild-type alleles of Nramp2 segregating in the Belgrade colony. For comparison, a full-length cDNA clone was also obtained from a rat cerebellum cDNA library. This clone contains an alternatively spliced C-terminal exon, yielding a protein 568 aa long, similar to the previously reported mouse sequence (GenBank accession no. L33415), but different from the published rat and human sequences (GenBank accession nos. AF008439 and L37347), as shown in Fig. 2. Several mouse expressed sequence tags (e.g., GenBank accession nos. AA463173 and W13403) and a splice isoform we isolated from a mouse erythroleukemia (MEL) cell line have C termini similar to the rat and human clones. Full-length mouse and rat clones having this alternative splice site have predicted ORFs of 561 aa. Mouse versions of each of these splice isoforms are capable of stimulating iron uptake in transient transfection assays (M.A.S., unpublished data), but the physiological significance of these alternative exons is unknown. Consequently, we analyzed coding sequences for both forms from b RNA. Overall, the only consistent predicted amino acid difference observed between wild type and b mutant RT-PCR clones, including both alternative C-terminal exons, was a glycine-to-arginine mutation resulting from a G-to-A transition at codon 185 (G185R, Fig. 3). The presence of this mutation in the germ line was confirmed by sequencing genomic PCR products spanning TM4. The G185R amino acid change also differs from a rat cDNA clone isolated from the cerebellum library, the previously reported rat Nramp2 cDNA sequence, the wild-type rat genomic sequence, and the wild-type mouse and human sequences. Strikingly, the identical amino acid alteration occurs in Nramp2 in two independent alleles of the mouse mutation microcytic anemia (mk1J and mk2J, ref. 15). Amino acid and silent third-position differences between the rat and mouse cDNAs exclude the possibility that mk mouse Nramp2 sequences contaminated our rat RT-PCR analyses. The presence of this mutation in mice with a similar phenotype strongly supports the hypothesis that a defect in Nramp2 is also responsible for the Belgrade phenotype.
Figure 2.
Amino acid alignment of alternative Nramp2 C-terminal exons. Predicted amino acid sequences of alternatively spliced Nramp2 C-terminal exons from mouse and rat are indicated by boxes. Two forms, 568 aa and 561 aa, have been isolated from rat (M.D.F., GenBank accession no. AF029757 and ref. 17) and mouse (M.D.F., GenBank accession no. AF029758 and ref. 28). The human C terminus determined from a cDNA clone lacking N-terminal sequences (GenBank accession no. L37347) resembles the 561-aa form from rat and mouse.
Figure 3.
Belgrade rats have a glycine-to-arginine missense mutation in Nramp2. Multiple overlapping RT-PCR clones spanning the wild-type and b allele of Nramp2 were isolated and sequenced. A single polymorphism resulting in a glycine-to-arginine substitution at codon 185 was identified. The presence of the mutation, indicated by the arrow, was verified by sequencing genomic PCR clones of the region.
The G185R Mutation Abrogates Iron Uptake Mediated by Nramp2.
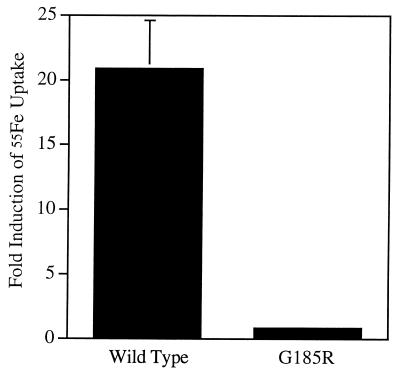
To assess the functional consequences of the G185R mutation, we examined the ability of the wild-type and G185R mutant alleles to stimulate iron uptake in transient transfection assays. HEK293T cells were transfected with expression plasmids containing the 568-aa form of the wild-type and b mutant Nramp2 proteins. At 48 hr after transfection, cells were assayed for iron uptake at pH 6.0 in the presence of the reducing agent, ascorbic acid. The assay is linear over the 20-min incubation period (M.A.S., unpublished data). The wild-type protein stimulated iron uptake approximately 20-fold compared with the antisense control construct (Fig. 4). Activity of the G185R mutant allele, however, did not exceed that of the antisense control plasmid in this assay. The loss of iron transport function observed in the G185R mutant allele supports a role for the normal allele in iron metabolism, indicates that the mutation is functionally significant, and supports the hypothesis that the mutation accounts for the b phenotype.
Figure 4.
The G185R mutation disrupts Nramp2-mediated iron uptake activity. HEK293T cells were transfected with expression plasmids containing wild-type or G185R mutant Nramp2 cDNA, and iron uptake activity was measured. Iron uptake is expressed as fold stimulation compared with cells transfected with plasmids containing the wild-type cDNA in an antisense orientation. Error bars indicate the standard deviation based on four independent experiments.
DISCUSSION
Although the roles of Tf and TfR in the transferrin cycle have been well characterized, many other fundamental aspects of iron metabolism are not well understood. Among them, the molecular mechanisms of two processes, transport of iron into the intestinal epithelial cell and out of the transferrin cycle endosome, have been particularly elusive. To understand the first of these transport steps, we sought to determine the molecular basis of the iron metabolic defect in mk mice. These animals have a defect in initial iron uptake into the duodenal epithelial cell, suggesting that the mk gene product acts at the apical surface of the enterocyte (12). Using a positional cloning approach, we recently identified Nramp2 as the defective gene and, on the basis of the structural features of the protein and the mk phenotype, postulated that Nramp2 was an iron transporter (15). Using an expression-cloning strategy in Xenopus oocytes, Gunshin et al. concurrently reported that Nramp2 promoted uptake of iron as well as other divalent cations, including manganese, cobalt, and zinc, and was up-regulated in the duodenum in response to iron deficiency (17). Taken together, these two studies strongly implicate Nramp2 in intestinal iron and divalent cation uptake.
Previous bone marrow transplantation studies clearly demonstrated that mk mice had a hematopoietic defect in addition to an intestinal defect in iron metabolism (13). There was limited evidence to indicate that the functional deficit was in the red cell precursor (14), but the transferrin cycle had not been specifically evaluated in mk reticulocytes. Consequently, we wished to investigate the possibility that Nramp2 might also be the Tf cycle endosomal iron transporter in addition to the intestinal apical epithelial transporter.
In contrast to mk, the functional abnormality in Belgrade rat reticulocytes has been studied extensively (3–7, 18, 19). Investigation of the Tf cycle in these cells has shown that much of the endocytosed iron is returned to the extracellular space with Tf, indicative of a defect in transfer of iron from the endosome (refs. 5 and 6; M.A.R., unpublished data). Transport alterations are also seen with non-Tf bound iron (refs. 7, 8, 11, and 22; L.M.G., unpublished results), suggesting that the defect is not in release from Tf, but in an iron transporter or a protein that regulates a transporter. The phenotypic similarity between the two animals, functional evidence of a disrupted iron transporter in the Belgrade rat, and the identification of a mutation in the iron transporter Nramp2 in mk animals suggested that Nramp2 was also a candidate gene for the b mutation. Using genetic mapping techniques, we have demonstrated linkage between the b phenotype and Nramp2 in the rat and identified a functionally significant mutation in the b allele that is shared by the mk mouse. Given the strong documentation of an endosomal iron transport defect in the b rat, described above, these findings support the hypothesis that Nramp2 is the Tf cycle endosomal iron transporter in addition to an intestinal transporter. Inefficient transfer of iron from the Tf cycle endosome as is seen in the b rat is exactly the phenotype expected from disruption of the endosomal transporter.
In addition to Tf-dependent iron uptake, iron may also be transported through a Tf-independent mechanism. A number of different non-Tf bound iron uptake activities have been described in hematopoietic, epithelial, and mesenchymal cells, each having variable requirements for ATP or calcium and dependence on iron concentration (8, 20–27). Many share a requirement for iron in the ferrous (Fe+2) state and are thought to be mediated by cell surface proteins. As Nramp2 is expressed at a low level in many tissues (28), it is possible that the Tf-dependent and independent pathways share a common component, namely Nramp2.
Remarkably, the G185R mutation identified in the b rat is identical to that present in the two existent mk alleles (15). The association of this mutation with an anemic phenotype in two species verifies its identity as the causative gene in mk and b. In the mouse, the mutation occurs at a CpG dinucleotide, which could account for the recurrent G-to-A transition seen in the two alleles of mk. However, in the rat, a TpG is present at this position, which is not prone to mutation. The identification of a unique recurrent mutation raises the possibility that the phenotypes seen in the mk mouse and the b rat are peculiar to a glycine-to-arginine mutation at this site. Perhaps this alteration results in a protein whose transporter activity is diminished to such an extent that a grossly anemic phenotype is readily recognizable, but not so severe that it is lethal. In the assay system used, no discernible residual iron uptake activity is seen with the G185R rat mutant; however, the alteration in Nramp2 function may be less severe in vivo. The mutation could also result in defective posttranslational processing of the protein leading to an absence of cell surface or endosomal expression. Alternatively, the G185R mutation could interfere with interaction of Nramp2 with another component of the transport apparatus, altering its function.
Divalent cations other than iron can also be transported by Nramp2 (17). Consequently, the b and mk phenotypes may not only be due to functional iron deficiency but also to derangements in manganese, cobalt, or zinc metabolism. These cations have not been studied in the mk mouse, but b rats are reported to have widespread abnormalities in manganese metabolism, including impaired duodenal and reticulocyte uptake (29). Furthermore, the mk mouse suffers from a transient skin abnormality and hair loss with features resembling zinc deficiency (30). The effect of parenteral or enteral supplementation of manganese, cobalt, and zinc has not been investigated in these animals.
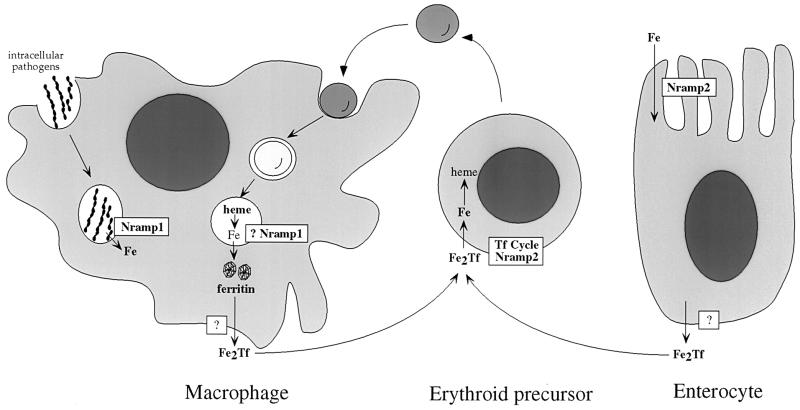
On the basis of the b and mk phenotypes, we propose a model for iron metabolism in mammals that incorporates Nramp2 (Fig. 5). Ferrous iron within the intestinal lumen is taken up into epithelial cells by apically expressed Nramp2. Intraepithelial iron is transferred to the serum by an unknown transporter and associates with serum apo-Tf. It is possible that a component of the basolateral transporter is mutated in the sex-linked anemia (sla) mouse, which has a defect in iron transfer from the duodenal epithelial cell into the serum (31). Diferric Tf is then taken up by receptor-mediated endocytosis, and the iron is mobilized from the acidified endosome by Nramp2. In the case of red blood cells, which contain the bulk of the body iron, iron is incorporated primarily into hemoglobin. Senescent red cells are phagocytosed by cells of the reticuloendothelial system, located primarily in the spleen, and iron is scavenged from heme. Given the high level of macrophage expression and intracellular localization in phagosomes of the homologous protein Nramp1, which can also transport iron (17), it is possible that Nramp1 transports iron scavenged from red cells out of phagosomes in macrophages. Subsequent transfer of iron back to the serum might also involve an Nramp protein.
Figure 5.
Proposed model of iron metabolism that incorporates Nramp proteins. Aspects of mammalian iron metabolism including absorption by intestinal epithelial cells, uptake by erythrocytes, and recycling by macrophages are depicted. Possible roles for Nramp2 and Nramp1 are based on phenotypes of mutant animals and functional studies, and are discussed in the text.
Nramp2 is the first mammalian iron transporter to be characterized on a molecular level. We propose that it is the iron transporter responsible for specific iron absorption in the intestine as well as mobilization of iron from endosomes during the Tf cycle. It is interesting to note that spontaneous missense changes in both Nramp2 and Nramp1 have occurred in rodents and each is associated with profound phenotypic effects; in addition to the changes in Nramp2 reported previously (15) and in this paper, a glycine-to-aspartic acid mutation in mouse Nramp1 has been shown to be responsible for susceptibility to infection by intracellular pathogens (32). Strikingly, these mutations in both Nramp1 and Nramp2 affect adjacent amino acid residues in predicted transmembrane domain 4 of the proteins. It is likely that disrupted endosomal cation transport also underlies the pathophysiology of the Nramp1 pathogen-sensitive phenotype. Given the phenotypes of rodents with mutations in Nramp proteins, it is likely that they serve largely nonredundant functions. Likewise, one might predict that a subset of human patients with congenital anemia might also harbor mutations in Nramp2. Indeed, isolated families with an apparently autosomal recessively inherited iron-deficiency anemia unresponsive to iron therapy have been described (33, 34). Given their strong phenotypic resemblance to the b rat and the mk mouse, the possibility exists that Nramp2 is also mutated in these congenitally anemic individuals.
Acknowledgments
We thank E. Neufeld for providing the rat cerebellum cDNA library, C. Trenor for handling sample shipments, and members of the Andrews and Garrick labs for helpful discussions. M.D.F. is supported by National Institutes of Health Grant K08 HL03600. L.M.G. and M.D.G. are supported by National Institutes of Health Grant R01 HL48690. N.C.A. is an Assistant Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- Tf
transferrin
- TfR
transferrin receptor
- NTA
nitrilotriacetic acid
- RT
reverse transcription
Footnotes
References
- 1.Bannerman R M. Fed Proc. 1976;35:2281. [PubMed] [Google Scholar]
- 2.Sladic-Simic D, Martinovich P N, Zivkovic N, Pavic D, Martinovic J, Kahn M, Ranney H M. Ann N Y Acad Sci. 1969;165:93–99. doi: 10.1111/j.1749-6632.1969.tb27779.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards J A, Garrick L M, Hoke J E. Blood. 1978;51:347–357. [PubMed] [Google Scholar]
- 4.Edwards J A, Sullivan A L, Hoke J E. Blood. 1980;55:645–648. [PubMed] [Google Scholar]
- 5.Bowen B J, Morgan E H. Blood. 1987;70:38–44. [PubMed] [Google Scholar]
- 6.Garrick M D, Gniecko K, Liu Y, Cohan D S, Garrick L M. J Biol Chem. 1993;20:14867–14874. [PubMed] [Google Scholar]
- 7.Farcich E A, Morgan E H. Am J Hematol. 1992;39:9–14. doi: 10.1002/ajh.2830390104. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson L L, Quail E A, Morgan E H. J Cell Physiol. 1995;162:181–190. doi: 10.1002/jcp.1041620204. [DOI] [PubMed] [Google Scholar]
- 9.Oates P S, Morgan E H. Am J Physiol. 1996;270:G826–32. doi: 10.1152/ajpgi.1996.270.5.G826. [DOI] [PubMed] [Google Scholar]
- 10.Garrick M, Scott D, Walpole S, Finkelstein E, Whitbred J, Chopra S, Trivikram L, Mayes D, Rhodes D, Cabbagestalk K, Oklu R, Sadiq A, Mascia B, Hoke J, Garrick L. Biometals. 1997;10:65–70. doi: 10.1023/a:1018370804882. [DOI] [PubMed] [Google Scholar]
- 11.Russell E S, Nash D J, Bernstein S E, Kent E L, McFarland E C, Matthews S M, Norwood M S. Blood. 1970;35:838–850. [PubMed] [Google Scholar]
- 12.Edwards J A, Hoke J E. Proc Soc Exp Biol Med. 1972;141:81–84. doi: 10.3181/00379727-141-36720. [DOI] [PubMed] [Google Scholar]
- 13.Harrison D E. Blood. 1972;40:893–901. [PubMed] [Google Scholar]
- 14.Edwards J A, Hoke J E. Blood. 1975;46:381–388. [PubMed] [Google Scholar]
- 15.Fleming M D, Trenor C C I, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 16.Manly K F. Mamm Genome. 1993;4:303–313. doi: 10.1007/BF00357089. [DOI] [PubMed] [Google Scholar]
- 17.Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 18.Garrick L M, Gniecko K, Hoke J E, al-Nakeeb A, Ponka P, Garrick M D. J Cell Physiol. 1991;146:460–465. doi: 10.1002/jcp.1041460317. [DOI] [PubMed] [Google Scholar]
- 19.Garrick L M, Gniecko K, Liu Y, Cohan D S, Grasso J A, Garrick M D. Blood. 1993;81:3414–3421. [PubMed] [Google Scholar]
- 20.Egyed A. Br J Haematol. 1988;68:483–486. doi: 10.1111/j.1365-2141.1988.tb04241.x. [DOI] [PubMed] [Google Scholar]
- 21.Sturrock A, Alexander J, Lamb J, Craven C M, Kaplan J. J Biol Chem. 1990;265:3139–3145. [PubMed] [Google Scholar]
- 22.Kaplan J, Jordan I, Sturrock A. J Biol Chem. 1991;266:2997–3004. [PubMed] [Google Scholar]
- 23.Hamazaki S, Glass J. Exp Hematol. 1992;20:436–441. [PubMed] [Google Scholar]
- 24.Inman R S, Wessling-Resnick M. J Biol Chem. 1993;268:8521–8528. [PubMed] [Google Scholar]
- 25.Randell E W, Parkes J G, Olivieri N F, Templeton D M. J Biol Chem. 1994;269:16046–16053. [PubMed] [Google Scholar]
- 26.Inman R S, Coughlan M M, Wessling-Resnick M. Biochemistry. 1994;33:11850–11857. doi: 10.1021/bi00205a022. [DOI] [PubMed] [Google Scholar]
- 27.Jordan I, Kaplan J. Biochem J. 1994;302:875–879. doi: 10.1042/bj3020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunheid S, Cellier M, Vidal S, Gros P. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- 29.Chua A C, Morgan E H. J Comp Physiol. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- 30.Russell E S, McFarland E C, Kent E L. Transplant Proc. 1970;2:144–151. [PubMed] [Google Scholar]
- 31.Bannerman R M, Edwards J A, Pinkerton P H. In: Progress in Hematology. Brown E B, editor. New York: Grune & Stratton; 1973. pp. 131–179. [PubMed] [Google Scholar]
- 32.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan G R, Sheehan R G. J Pediatr. 1981;98:723–728. doi: 10.1016/s0022-3476(81)80831-1. [DOI] [PubMed] [Google Scholar]
- 34.Hartman K R, Barker J A. Am J Hematol. 1996;51:269–275. doi: 10.1002/(SICI)1096-8652(199604)51:4<269::AID-AJH4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]