Abstract
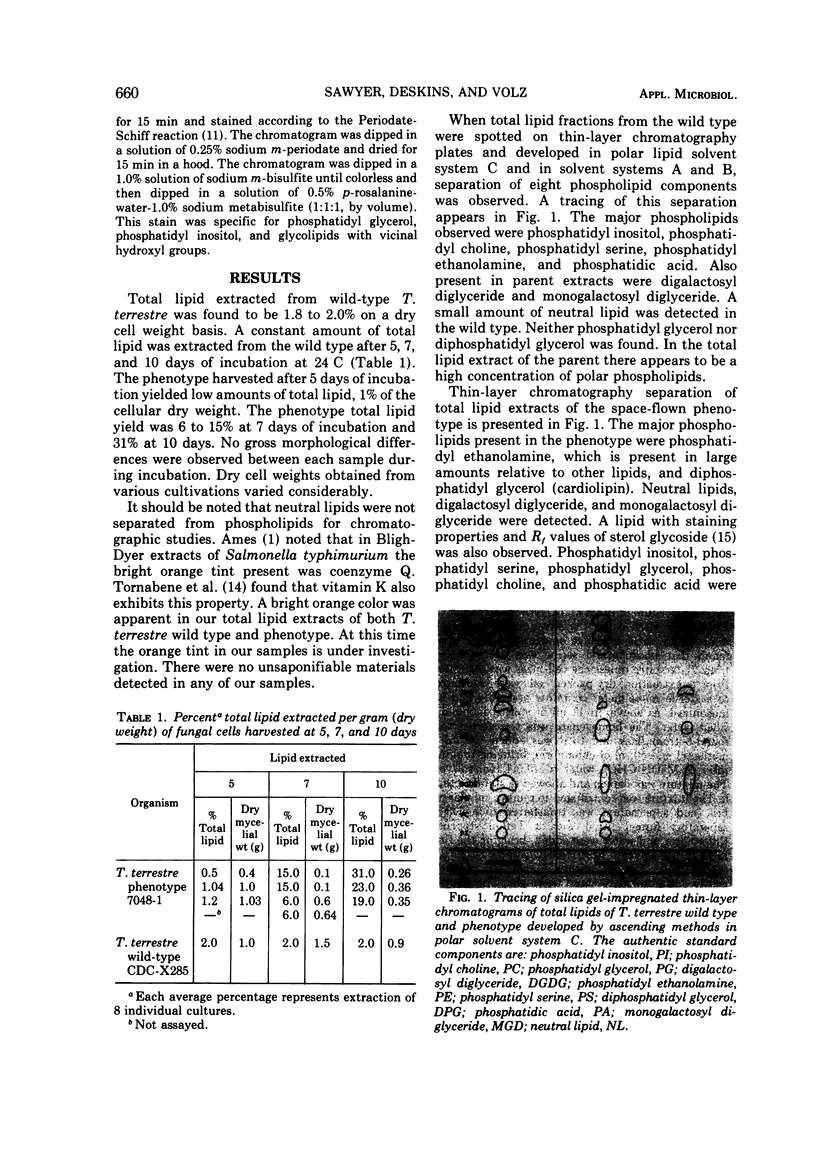
Total lipid extracted from wild-type Trichophyton terrestre CDC-X285 was found to be 2.0% of the dry cell weight. The total lipid contained the following phospholipid components identified by silicic acid-impregnated thin-layer and paper chromatography: phosphatidyl inositol, phosphatidyl choline, phosphatidyl serine, and phosphatidic acid. The total lipid extracted from the phenotype T. terrestre 7048-1 isolated from the Apollo 16 Microbial Ecology Evaluation Device (MEED) was found to vary according to the time at which the phospholipids were extracted. The Trichophyton phenotype was selected from a cuvette housed in the MEED exposed to specific space parameters including ultraviolet light of known wavelengths and energy levels in deep space. The phospholipid components identified in the phenotype were phosphatidyl ethanolamine and cardiolipin. The major lipid fraction was composed of digalactosyl diglyceride and monogalactosyl diglyceride. An unusual lipid was detected in the phenotype, which appeared to be sterol glycoside.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Das S. K., Banerjee A. B. Phospholipids of Trichophyton rubrum. Sabouraudia. 1974 Nov;12(3):281–286. [PubMed] [Google Scholar]
- Dublin M., Volz P. A., Bulmer G. S. The antifungal activity of normal and host-compromised saliva on spaceflight fungal phenotypes. Mycopathol Mycol Appl. 1974 Dec 18;54(4):499–516. doi: 10.1007/BF02050055. [DOI] [PubMed] [Google Scholar]
- Dublin M., Volz P. A. Space related research in mycology concurrent with the first decade of manned space exploration. Space Life Sci. 1973 Apr;4(2):223–230. doi: 10.1007/BF00924469. [DOI] [PubMed] [Google Scholar]
- Ikawa M. Bacterial phosphatides and natural relationships. Bacteriol Rev. 1967 Mar;31(1):54–64. doi: 10.1128/br.31.1.54-64.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Taylor G. R. Space microbiology. Annu Rev Microbiol. 1974;28(0):121–137. doi: 10.1146/annurev.mi.28.100174.001005. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Kates M., Gelpi E., Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969 May;10(3):294–303. [PubMed] [Google Scholar]
- Tornabene T. G. Lipid composition of selected strains of Yersinia pestis and Yersinia pseudotuberculosis. Biochim Biophys Acta. 1973 May 24;306(2):173–185. doi: 10.1016/0005-2760(73)90223-3. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Ogg J. E. Chromatographic studies of the lipid components of Vibrio fetus. Biochim Biophys Acta. 1971 Jul 13;239(2):133–141. doi: 10.1016/0005-2760(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Volz P. A., Dublin M. Filamentous fungi exposed to spaceflight stresses including known levels of ultraviolet irradiations. Space Life Sci. 1973 Sep-Dec;4(3):402–414. doi: 10.1007/BF00930352. [DOI] [PubMed] [Google Scholar]
- Volz P. A., Hsu Y. C., Hiser J. L., Veselenak J. M., Jerger D. E. The Microbial Ecology Evaluation Device Mycology spaceflight studies of Apollo 16. Mycopathol Mycol Appl. 1974 Nov 10;54(2):221–233. doi: 10.1007/BF02050043. [DOI] [PubMed] [Google Scholar]
- Weiss B., Stiller R. L., Jack R. C. Sphingolipids of the fungi Phycomycetes blakesleeanus and Fusarium lini. Lipids. 1973 Jan;8(1):25–30. doi: 10.1007/BF02533235. [DOI] [PubMed] [Google Scholar]





