Abstract
Ig class switching usually occurs as a consequence of cognate interactions between antigen-specific B cells and CD4+ αβ T cells. Vesicular stomatitis virus (VSV) infection of immunocompetent mice induces a rapid T-independent neutralizing IgM response followed by a long-lived T-dependent IgG response. Surprisingly, αβ T cell-deficient (TCRα−/−) mice also produced neutralizing IgG antibodies when infected with live VSV or with a recombinant vaccinia virus expressing the VSV glycoprotein (Vacc-IND-G), but not when immunized with UV-inactivated VSV (UV-VSV). The neutralizing IgG responses did not require the presence of NK cells or complement, but were crucially dependent on IFN-γ and were predominantly of the IgG2a isotype. IgG production depended on residual CD3+ non-αβ T cell populations present in the TCRα−/− mice, which produced IFN-γ upon in vitro stimulation. A key role for γδ T cells was confirmed by the fact that TCRβ−/− mice also generated strong neutralizing IgG responses to VSV, whereas TCRβ−/−δ−/− mice produced very low titers. The neutralizing IgG responses of TCRα−/− mice were accompanied by the development of memory B cells, but not by antigen-specific germinal center (GC) formation. Thus, during viral infection of αβ T cell-deficient mice, γδ T cells may provide the signals that are required for isotype switching.
Vesicular stomatitis virus (VSV) infection of immunocompetent mice induces a rapid neutralizing IgM response, which occurs independently of T cell help, followed by production of neutralizing IgG antibodies that are strictly dependent on T cell help (1). Although VSV also induces cytotoxic T lymphocytes (2, 3), the neutralizing IgG response seems to be crucial for recovery from primary infections (4). The generation of high-affinity antibodies and class switching from IgM to IgG is usually the result of cognate interactions between antigen-specific B cells and CD4+ αβ T cells, the latter secreting cytokines that regulate Ig isotype switching (5–7). These T-dependent B cell responses are accompanied by the formation of specialized microenvironments, germinal centers (GC), within which somatic mutation and affinity maturation occur (8, 9).
However, evidence is growing that there may be alternative, albeit less efficient, mechanisms for the generation of IgG antibody responses. For example, mice that have been rendered T cell-deficient by targeted disruption of the TCRα gene (TCRα−/−) exhibit elevated serum IgG levels and enhanced numbers of GC in comparison to TCRα+/− littermates (10–12). Furthermore, athymic nude mice, although generally having low levels of IgG antibodies and few GC, can produce VSV-specific IgG antibodies (13) and can clear intracerebral sindbis virus infection (14). Finally, in the case of polyoma virus infection of T cell-deficient mice, it was recently shown that B cells alone were capable of mounting a protective T cell-independent antiviral antibody response, including the production of IgG antibodies (15).
In this study, we have examined the anti-viral antibody responses of T cell-deficient mice infected with VSV and have characterized the factors involved in the generation of T-independent anti-viral IgG responses.
MATERIALS AND METHODS
Mice.
C57BL/6 (H-2b) mice were from the Institut für Labortierkunde (Zürich, Switzerland). Mice with a mutant T cell receptor α gene (TCRα−/−) (16) were obtained from M. J. Owen, (Imperial Cancer Research Fund, London), and TCRβ−/− mice (17) were obtained from U. Steinhoff, (Max-Planck-Institut für Infektionsbiologie, Berlin). Mice with nonfunctional TCR β and δ genes (TCRβ−/−δ−/−) (17) were obtained from The Jackson Laboratory. All T cell-deficient strains had been backcrossed to C57BL/6 (H-2b) mice.
Viruses, Immunization, Measurement of VSV-Specific Antibody Responses, and Immunohistology.
VSV Indiana (VSV-IND, Mudd–Summers isolate) was used in this study and was propagated and UV-inactivated (UV-VSV) as described previously (18, 19). Recombinant vaccinia viruses expressing the VSV-IND surface glycoprotein (Vacc-IND-G) or lymphocytic choriomeningitis virus nucleoprotein (Vacc-LCMV-NP) or murine interferon γ (Vacc-IFN-γ) were prepared as described previously (20, 21).
Mice were immunized i.v. with 2 × 106 pfu of infectious VSV-IND, UV-VSV, or Vacc-IND-G. VSV-neutralizing IgM and IgG antibody titers were assayed as described (22, 23). Mean titers from groups of three mice are shown, and intragroup variations were ≤2 titer steps. The isotype distribution of VSV-specific IgG was determined by ELISA on VSV-coated plates (24). Immunohistological analysis of frozen spleen sections was performed as described previously (25).
Reagents for in Vivo Depletions.
To deplete T cell or natural killer (NK) cell populations in vivo, mice were injected i.p. with 0.5 mg anti-Thy1.2 mAb (26) or with polyclonal rabbit anti-AsialoGM1 antiserum (Waco) on days 3 and 1 before immunization. Antibody treatments were repeated on days 5 and 11 after immunization. IFN-γ was depleted by daily i.p. injection of 0.2 ml of a polyclonal sheep antiserum (27), commencing 24 h before immunization. For complement depletion, 1.2 μg purified cobra venom factor (CVF) (Naja naja, Sigma) in 200 μl balanced salt solution (BSS) was injected twice i.p., with an interval of 8–10 h, and mice were immunized 16 h later (28). Complement depletion was repeated on days 5 and 11 after immunization.
Intracellular Cytokine Staining.
Thy1.2+ spleen cells were obtained to a purity of ≥95% by magnetic antibody cell sorting (MACS) purification with anti-Thy1.2 microbeads (Miltenyi Biotech, Germany). Intracellular cytokine staining for interleukin (IL)-4 and IFN-γ production was assayed as described (29, 30), by using a Cytostain kit (PharMingen). T cell subsets were distinguished by staining beforehand with biotinylated anti-TCRβ or anti-TCRδ (both PharMingen), followed by tricolor-streptavidin (Caltag, South San Francisco, CA). Samples were analyzed by using a FACScan (Becton Dickinson).
Adoptive Transfer of Memory B Cells.
The presence of memory B cells was assessed by a modification of a previously described adoptive transfer system (31). Briefly, 107 MACS-purified B220+ B cells isolated from TCRα−/− mice that had been immunized 30–40 days previously with 2 × 106 pfu VSV-IND were adoptively transferred i.v. into naive TCRα−/− recipients. Two hours later the recipients were challenged with 2 × 106 pfu UV-VSV i.v. Sera samples were isolated at various time points after challenge, and VSV neutralizing IgG titers were measured as described.
RESULTS
T Cell-Deficient Mice Produce Neutralizing IgG Responses to VSV.
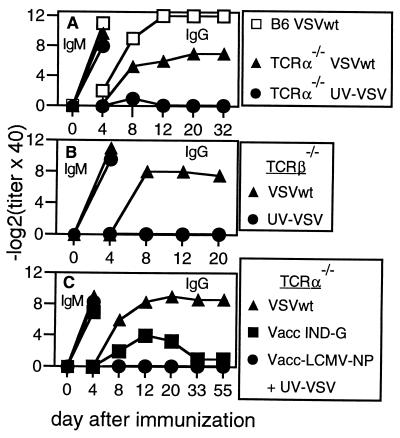
To explore whether mice congenitally deficient in T cells were capable of mounting anti-viral IgG responses, we examined the neutralizing antibody responses of TCRα−/− mice after immunization with either live or inactivated VSV. TCRα−/− mice produced high titers of neutralizing IgM antibodies early after challenge with live VSV or with UV-inactivated VSV (Fig. 1A), responses known to occur independently of T cell help (24). Surprisingly however, TCRα−/− mice given live VSV also developed neutralizing IgG antibodies, whereas those given inactivated VSV did not (Fig. 1A). Similar VSV-neutralizing IgG responses were observed in TCRβ−/− mice given live VSV (Fig. 1B). To analyze whether mechanisms activated during live viral infection were responsible for the class switching, we infected TCRα−/− mice with a recombinant vaccinia virus expressing the VSV glycoprotein (Vacc-IND-G). Vacc-IND-G also induced low levels of VSV-specific neutralizing IgG antibodies in TCRα−/− mice (Fig. 1C), even though the VSV-G is not present in the envelope of the recombinant vaccinia virus (32). In addition, TCRα−/− mice injected with a recombinant vaccinia expressing an irrelevant antigen (Vacc-LCMV-NP) together with UV-inactivated VSV produced VSV-neutralizing IgM antibodies but no IgG (Fig. 1C). These results confirmed that for the induction of neutralizing IgG, live virus infection was more important than the ability to efficiently crosslink B cell receptors and suggested that the additional factors inducing class switching must be present within the local microenvironment.
Figure 1.
VSV-neutralizing antibody responses of T cell-deficient mice. C57BL/6 mice (A), TCRα−/− mice (A and C), or TCRβ−/− mice (B) were immunized with 2 × 106 pfu of live or UV-inactivated VSV i.v. (A and B), or with 2 × 106 pfu of Vacc-IND-G i.v. or 2 × 106 pfu of both Vacc-LCMV-NP and UV-inactivated VSV i.v. (C). One of three similar experiments is shown.
T Cell-Independent VSV-Neutralizing IgG Responses Occur Independently of NK Cells or Complement Activation.
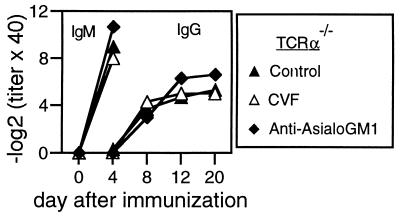
Fragments generated after activation of the third complement component C3 can bind to complement receptors on the surface of B cells and greatly augment B cell responsiveness (33–36). Because VSV seems able to bind C3 (37), we assayed VSV neutralizing responses of TCRα−/− mice after depletion of C3 with cobra venom factor (CVF) (28). However, CVF treatment had no effect on the production of VSV-neutralizing IgG (Fig. 2) after live virus infection. NK cell activity may be an important mechanism in the early control of virus replication and spread (38, 39). Furthermore, evidence exists that NK cells may provide B cell “help” for the production of IgG antibodies against T-independent (TI) polysaccharide antigens (40). Although NK cell activity was enhanced during VSV infection (data not shown), depletion of NK cells by anti-AsialoGM1 treatment had no effect on the development of VSV-neutralizing IgG responses in TCRα−/− mice (Fig. 2). Thus, neither complement nor NK cells were limiting for isotype switching in TCRα−/− mice.
Figure 2.
VSV-neutralizing IgG responses are not dependent on complement or NK cells. TCRα−/− mice were treated i.p. with CVF or with anti-Asialo GM1 antibodies before immunization with 2 × 106 pfu of live VSV i.v. Treatments were repeated on days 5 and 11. One of two similar experiments is shown.
Non-αβ T Cells Provide “Help” for VSV-Neutralizing IgG Responses via the Production of IFN-γ.
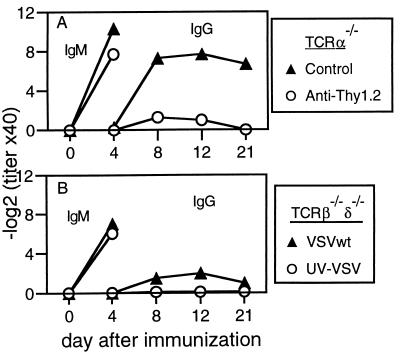
Although devoid of conventional αβ T cells, TCRα−/− mice contain elevated numbers of γδ T cells and a population of CD3+ cells that express the TCRβ chain (TCRα−β+) (16, 41). Depletion of Thy1.2+ cells almost completely abrogated the neutralizing IgG response of TCRα−/− mice (Fig. 3A), showing that residual non-αβ T cells were required. That TCRβ−/− mice, which do not possess TCRα−β+ cells, also produced neutralizing IgG (Fig. 1B) suggested that γδ T cells played a crucial role in this class switching. This was confirmed by analysis of double-mutant TCRβ−/−δ−/− mice (17), which produced only very low titers of neutralizing IgG antibodies after VSV infection (Fig. 3B).
Figure 3.
γδ T cells are required for VSV-neutralizing IgG responses in αβ T cell-deficient mice. (A) TCRα−/− mice were treated i.p. with monoclonal antibodies against Thy1.2 on days 3 and 1 before immunization with 2 × 106 pfu of live VSV i.v. Antibody treatment was repeated on days 5 and 11. (B) TCRβ−/−δ−/− mice were immunized with 2 × 106 pfu of live or UV-inactivated VSV i.v. One of three similar experiments is shown.
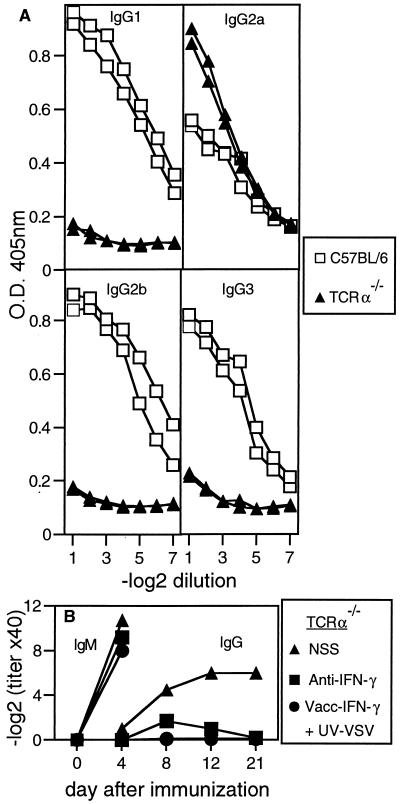
Interestingly, whereas immunocompetent mice infected with VSV produced VSV-specific IgG of all subclasses (Fig. 4A), TCRα−/− mice produced very low levels of IgG1, IgG2b, and IgG3, but strikingly high levels of IgG2a antibodies (Fig. 4A). IFN-γ is known to promote switching to IgG2a production (5, 7), and depletion of IFN-γ had a profound inhibitory effect on the production of VSV-neutralizing IgG antibodies by TCRα−/− mice (Fig. 4B) and reduced the levels of serum VSV-specific IgG2a (data not shown). The need for linkage between the B cell activator and the cytokine to promote isotype switching was illustrated by the finding that TCRα−/− mice infected with a recombinant vaccinia expressing IFN-γ together with UV-inactivated VSV produced neutralizing IgM antibodies but not IgG (Fig. 4B).
Figure 4.
Isotype distribution and IFN-γ dependence of VSV-specific antibodies produced by TCRα−/− mice. (A) The isotypes of VSV-specific IgG present in the sera of C57BL/6 and TCRα−/− mice 20 days after immunization with 2 × 106 pfu of live VSV i.v. were determined by ELISA. Each line represents an individual mouse, and one of three similar experiments is shown. (B) TCRα−/− mice were treated daily with polyclonal sheep anti-IFN-γ antisera or normal sheep serum (NSS) from day 1 before immunization with 2 × 106 pfu of live VSV i.v. An additional group of untreated TCRα−/− mice received 2 × 106 pfu of Vacc-IFN-γ together with 2 × 106 pfu of UV-inactivated VSV i.v. One of two similar experiments is shown.
Analysis of intracellular cytokine expression by Thy1.2+ spleen cells showed that around 97% of Thy1.2+ C57BL/6 spleen cells expressed CD3, with the vast majority (95%) being αβ T cells (Fig. 5A). After mitogenic stimulation, 17% of these cells produced IFN-γ whereas none produced IL-4 (Fig. 5B). In TCRα−/− mice, around 70% of Thy1.2+ spleen cells were CD3+, with 10% being TCRβ+ and 60% expressing the γδ TCR (Fig. 5 C and E). Of the TCRα−β+ cells, 43% expressed IFN-γ and 7% produced IL-4 after in vitro stimulation (Fig. 5D). Among the γδ T cells, 66% produced IFN-γ and none produced IL-4 after in vitro stimulation (Fig. 5F). Thus, by considering the relative proportions of each subpopulation, we calculated that >90% of IFN-γ-producing cells present in TCRα−/− mice were γδ T cells.
Figure 5.
Cytokine production by non-αβ T cells from TCRα−/− mice. Splenic Thy1.2+ cells purified from C57BL/6 (A and B) or TCRα−/− mice (C–F) were stimulated with PMA and ionomycin for 4 h in vitro. Surface CD3 and TCR expression (A, C, and E) and intracellular IL-4 and IFN-γ production (B, D, and F) were assessed by FACS. Intracellular cytokine expression patterns of gated populations of TCRα−β+ cells (D) and γδ T cells (F) from TCRα−/− mice are shown. One of two similar experiments is shown.
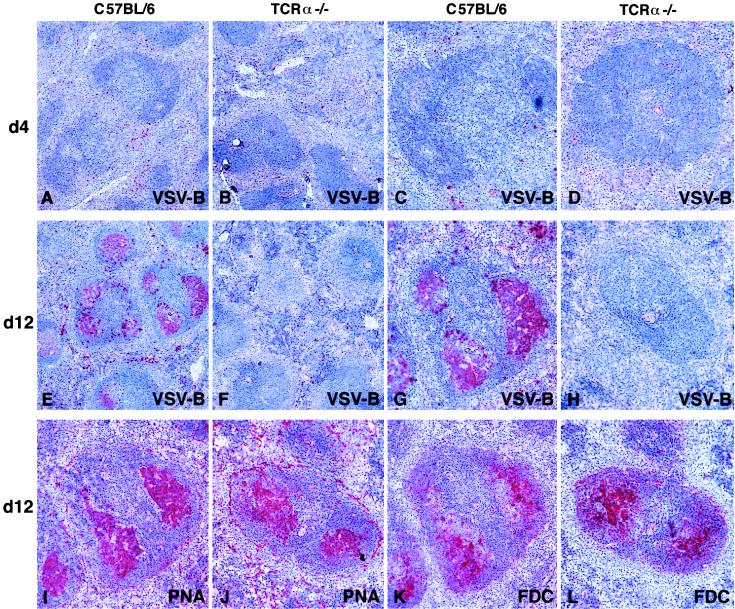
TCRα−/− Mice Mount a Memory B Cell Response in the Absence of Germinal Center Formation.
Germinal center formation and memory B cell development are generally assumed to be dependent on signals provided by CD4+ αβ T cells (8, 9). To assess memory B cell development, we adoptively transferred purified B cells from VSV-primed TCRα−/− mice into naive TCRα−/− mice and challenged the recipients with UV-inactivated VSV. Although naive TCRα−/− mice produced neutralizing IgM on challenge with UV-VSV, only those mice that received primed B cells generated neutralizing IgG antibodies (Fig. 6), confirming that memory B cells had been primed in TCRα−/− mice. To examine for GC formation, TCRα−/− or C57BL/6 mice were infected with VSV, spleens isolated at various time points thereafter and subjected to immunohistological analysis (25). In both C57BL/6 and TCRα−/− mice 4 days after infection, VSV-specific B cells were predominantly located in the red pulp and marginal zone areas, with a few cells scattered through the B cell follicles (Fig. 7 A–D). By day 12 after infection, VSV-specific GC could clearly be detected in C57BL/6 mice, as evidenced by the colocalization of VSV-specific B cells, PNA+ B cells and follicular dendritic cells (FDC) (Fig. 7 E, G, I, and K). However, VSV-specific GC were not detected in any of the TCRα−/− mice examined and although some GC were observed, the few VSV-specific B cells present were found scattered in the follicles and red pulp (Fig. 7 F, H, J, and L). Identical results were observed in spleens isolated 20 days and 32 days after infection (data not shown).
Figure 6.
Neutralizing VSV-specific IgG memory responses after adoptive transfer of B cells from VSV-primed TCRα−/− mice. Purified splenic B cells (107) from TCRα−/− mice that had been immunized with 2 × 106 pfu of live VSV i.v. 1 month previously were adoptively transferred into naive TCRα−/− recipients i.v. Twenty-four hours later, recipients were challenged with 2 × 106 pfu of UV-inactivated VSV i.v. Control mice received either UV-VSV only, or primed B cells only. One of two similar experiments is shown.
Figure 7.
Lack of VSV-specific germinal center formation in TCRα−/− mice. C57BL/6 mice (A, C, E, G, I, and K) and TCRα−/− mice (B, D, F, H, J, and L) were immunized with 2 × 106 pfu of live VSV i.v. and spleen sections at the indicated time points were analyzed by immunohistochemistry. The stained quality is indicated in each panel: VSV-B, VSV-binding B cells; PNA, peanut hemagglutinin binding; FDC, follicular dendritic cells. Serial adjacent sections of the same follicle are shown from C57BL/6 (G, I, and K) and TCRα−/− (H, J, and L) mice. Original magnifications: ×50 (A, B, E, and F); ×100 (C, D, G, H, I, J, K, and L). Representative results from groups of two to three mice are shown. Similar results were obtained in spleen sections taken on days 20 and 32 after immunization and in three separate experiments.
DISCUSSION
This study demonstrates that Ig class switching and memory B cell development can occur after viral infection independently of conventional αβ T cell help and in the absence of specific GC formation. These findings contrast with previous observations that neutralizing IgG responses to VSV were strictly dependent on CD4+ αβ T cells (1), but parallel results obtained using athymic nude mice after infection with VSV (13) and T cell-deficient mice infected with polyoma virus, where B cells were capable of generating protective IgM and IgG antibody responses in the absence of T cells (15). Similar results have also been reported for the T-independent antigen TNP-Brucella abortis, where nude mice produced higher levels of TNP-specific IgG than immunocompetent mice depleted of CD4+ T cells (40). These paradoxical observations highlight differences between the effects of acute depletion of T cells of normal mice versus the development of the immune system in mice congenitally deficient in T cells, which favors the development of compensatory mechanisms of innate immunity (41, 42). Importantly, the levels of neutralizing IgG elicited by VSV infection of TCRα−/− mice were about 16-fold lower than those elicited in immunocompetent mice. Thus, isotype switching occurs considerably more efficiently in the presence of CD4+ αβ T cells; the small amounts of neutralizing IgG that may have been generated via alternative mechanisms therefore may usually be masked.
Interestingly, neutralizing IgG antibodies were only induced after live viral infection of TCRα−/− mice, but not after injection of inactivated VSV. This was not due to inefficient B cell triggering, because comparable titers of neutralizing IgM antibodies were elicited by inactivated VSV. Because VSV does not measurably replicate extraneuronally in adult mice (43) it is unlikely that the observed differences were due to differences in antigen dose. Recombinant vaccinia virus expressing the VSV glycoprotein also induced neutralizing IgG in TCRα−/− mice, although the titers were around 16- to 32-fold lower than those induced by live VSV. Because the recombinant vaccinia does not express the VSV-G in its envelope (32), these results indicate that expression of VSV-G in a highly organized, repetitive form on the surface of VSV virions greatly augmented isotype switching. Importantly, polyoma virus, which is also able to induce neutralizing IgG responses in T cell-deficient mice, also has a highly organized, repetitive structure (44).
IFN-γ was an essential factor required for isotype switching in TCRα−/− mice, because depletion of IFN-γ drastically reduced the levels of VSV-neutralizing IgG. Our results suggest that the IFN-γ must be produced in close proximity to the B cell activator, because coinjection of recombinant vaccinia virus expressing either an irrelevant antigen (LCMV-NP) or IFN-γ, together with UV-inactivated VSV, did not induce isotype switching. Therefore, some “linked recognition” or linked bystander event involving either the B cell alone or interaction with another cell must occur. The predominant isotype present in TCRα−/− mice was IgG2a, the production of which is enhanced by IFN-γ (5, 7). Previous studies have also found a striking preponderance of IgG2a antibodies after a range of viral infections (45, 46), indicating that viral infection selectively triggers mechanisms that promote the production of this isotype. Our findings, together with those showing that IgG2a also predominates in nude mice infected with VSV (13) and in T cell-deficient mice infected with polyoma virus (15), show that viral infection can induce switching to IgG2a independently of αβ T cell help. Because more than 90% of IFN-γ-producing Thy1.2+ cells in TCRα−/− mice were γδ T cells and because TCRβ−/− mice mounted similar VSV-neutralizing IgG responses whereas double mutant TCRβ−/−δ−/− mice did not, it appears that most of the class switching to IgG2a was supported by γδ T cells.
Surprisingly, the VSV-neutralizing IgG responses of TCRα−/− mice were not accompanied by antigen-specific GC formation, although we were able to confirm (12) that naive TCRα−/− mice contained higher numbers of GC than naive C57BL/6 mice (data not shown). These findings are similar to recent observations with gene-targeted mice deficient in tumor necrosis factor, leukotriene, or tumor necrosis factor-receptor I, all of which retain the capacity to mount IgG antibody responses in the absence of GC formation (47–49). It has also been proposed that the development of memory B cells occurs within B cell follicles and may be facilitated by cognate interactions between αβ T cells and B cells (8, 9). Our results indicate that there is an alternative, albeit less efficient, pathway of B cell activation and maturation, which can occur independently of αβ T cells and does not require the formation of GC.
Acknowledgments
We thank Lenka Vlk for the immunohistological analysis, Norbert Wey for the photographs, Alana Althage for sublime technical assistance, and Thomas Fehr, Annette Oxenius, and Peter Aichele for helpful suggestions and for critically reviewing the manuscript. This work was supported by the Swiss National Science Foundation and the Kanton Zürich.
ABBREVIATIONS
- VSV-IND
vesicular stomatitis virus serotype Indiana
- UV-VSV
UV light-inactivated VSV
- Vacc-IND-G
recombinant vaccinia virus-expressing VSV-IND glycoprotein
- IL
interleukin
- IFN
interferon
References
- 1.Leist T P, Cobbold S P, Waldmann H, Aguet M, Zinkernagel R M. J Immunol. 1987;138:2278–2281. [PubMed] [Google Scholar]
- 2.Rosenthal K L, Zinkernagel R M. J Immunol. 1980;124:2301–2308. [PubMed] [Google Scholar]
- 3.Lefrancois L, Lyles D S. J Immunol. 1982;130:1408–1412. [PubMed] [Google Scholar]
- 4.Bachmann M F, Althage A, Kündig T M, Kalberer C P, Hengartner H, Zinkernagel R M. J Immunol. 1994;152:4235–4241. [PubMed] [Google Scholar]
- 5.Finkelman F D, Holmes J, Katona I M, Urban J, Beckmann M P, Park L S, Scholley K A, Coffman R L, Mosmann T R, Paul W E. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 6.Parker D C. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 7.Snapper C M, Mond J J. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y J, Johnson G D, Gordon J, MacLennan I C M. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan I C M. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 10.Wen L, Roberts S J, Viney J L, Wong F S, Mallick C, Findly C, Peng Q, Craft J E, Owen M J, Hayday A C. Nature (London) 1994;369:654–658. doi: 10.1038/369654a0. [DOI] [PubMed] [Google Scholar]
- 11.Wen L, Pao W, Wong F S, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen M J, Hayday A C. J Exp Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dianda L, Gulbranson-Judge A, Pao W, Hayday A C, MacLennan I C M, Owen M J. Eur J Immunol. 1996;26:1603–1607. doi: 10.1002/eji.1830260729. [DOI] [PubMed] [Google Scholar]
- 13.Freer G, Burkhart C, Ciernik I, Bachmann M F, Hengartner H, Zinkernagel R M. J Virol. 1994;68:3650–3655. doi: 10.1128/jvi.68.6.3650-3655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyor W R, Moench T R, Griffin D E. J Neuroimmunol. 1989;24:207–215. doi: 10.1016/0165-5728(89)90118-5. [DOI] [PubMed] [Google Scholar]
- 15.Szomolyani-Tsuda E, Welsh R. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philpott K L, Viney J L, Kay G, Rastan S, Gardiner E M, Chae S, Hayday A C, Owen M J. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaenisch R, Hooper M L, Tonegawa S. Nature (London) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 18.McCaren L, Holland J J, Syverton J T. J Exp Med. 1959;109:475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachmann M F, Kündig T M, Kalberer C, Hengartner H, Zinkernagel R M. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackett M, Yilma T, Rose J K, Moss B. Science. 1985;227:433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- 21.Hany M, Oehen S, Schulz M, Hengartner H, Mackett M, Bishop D H L, Zinkernagel R M. Eur J Immunol. 1989;19:417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- 22.Wagner R R, Snyder R M, Yamazaki S. J Virol. 1970;5:548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott D W, Gershon R K. Clin Exp Immunol. 1970;6:13–18. [PMC free article] [PubMed] [Google Scholar]
- 24.Charan S, Zinkernagel R M. J Immunol. 1986;136:3057–3061. [PubMed] [Google Scholar]
- 25.Bachmann M F, Odermatt B, Hengartner H, Zinkernagel R M. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opitz H G, Opitz U, Hewlett G, Schlumberger H D. Immunobiology. 1982;160:438–453. doi: 10.1016/S0171-2985(82)80007-7. [DOI] [PubMed] [Google Scholar]
- 27.Leist T P, Eppler M, Zinkernagel R M. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehr T, Bachmann M F, Bluethmann H, Kikutani H, Hengartner H, Zinkernagel R M. Cell Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- 29.Assenmacher M, Schmitz J, Radbruch A. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 30.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O’Garra A. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmann M F, Kündig T M, Odermatt B, Hengartner H, Zinkernagel R M. J Immunol. 1994;153:3386–3397. [PubMed] [Google Scholar]
- 32.Bachmann M F, Hoffmann Rohrer U, Kündig T M, Bürki K, Hengartner H, Zinkernagel R M. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 33.Erdei A, Füst G, Gergely J. Immunol Today. 1991;12:332–337. doi: 10.1016/0167-5699(91)90011-H. [DOI] [PubMed] [Google Scholar]
- 34.Fearon D T, Carter R H. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey P W, Allison M E D, Akkaraju S, Goodnow C C, Fearon D T. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 36.DeFranco A L. Curr Biol. 1996;6:548–550. doi: 10.1016/s0960-9822(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 37.Beebe D P, Cooper N R. J Immunol. 1981;126:1562–1568. [PubMed] [Google Scholar]
- 38.Biron C A, Turgiss L R, Welsh R M. J Immunol. 1983;131:1539–1545. [PubMed] [Google Scholar]
- 39.Welsh R M, Brubaker J O, Vargas-Cortes M, O’Donnell C L. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mond J J, Lees A, Snapper C M. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 41.Mombaerts P, Mizoguchi E, Ljunggren H G, Iacomini J, Ishikawa H, Wang L, Grusby M J, Glimcher L H, Winn H J, Bhan A K, Tonegawa S. Int Immunol. 1994;6:1061–1070. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- 42.Viney J L, Dianda L, Roberts S J, Wen L, Mallick C A, Hayday A C, Owen M J. Proc Natl Acad Sci USA. 1994;91:11948–11952. doi: 10.1073/pnas.91.25.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner R R. In: Rhabdoviridae and Their Replication. Fields B N, Knipe D M, editors. Vol. 1. New York: Raven; 1990. pp. 867–882. [Google Scholar]
- 44.Stehle T, Yan Y, Benjamin T L, Harrison S C. Nature (London) 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]
- 45.Coutelier J P, Van der Logt J T M, Heessen F W A, Warnier G, Van Snick J V. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutelier J P, Van der Logt J T M, Heessen F W A, Van Snick J V. J Exp Med. 1988;168:2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y-J, Banchereau J. J Exp Med. 1996;184:1207–1211. doi: 10.1084/jem.184.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto M, Lo S F, Carruthers C J L, Min J, Mariathasan S, Huang G, Plas D R, Martin S M, Geha R S, Namh M H, Chaplin D D. Nature (London) 1996;382:462–466. doi: 10.1038/382462a0. [DOI] [PubMed] [Google Scholar]