Abstract
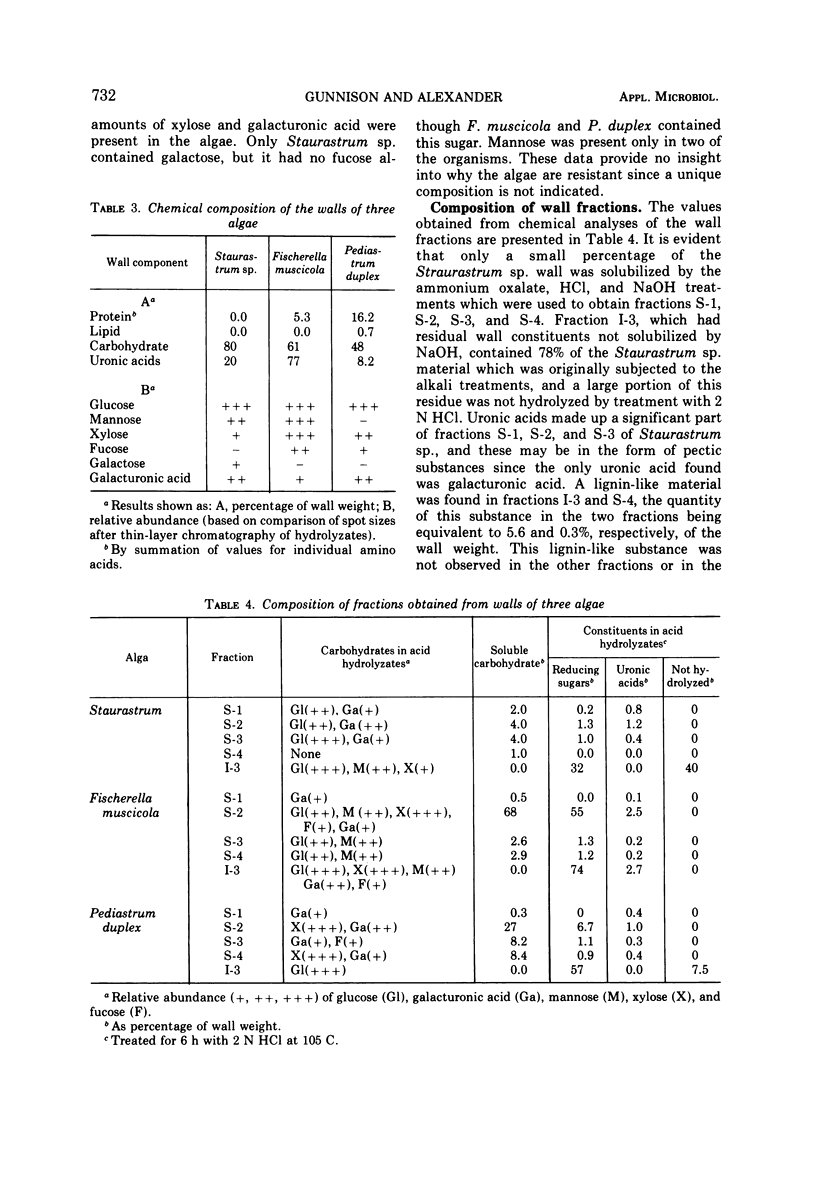
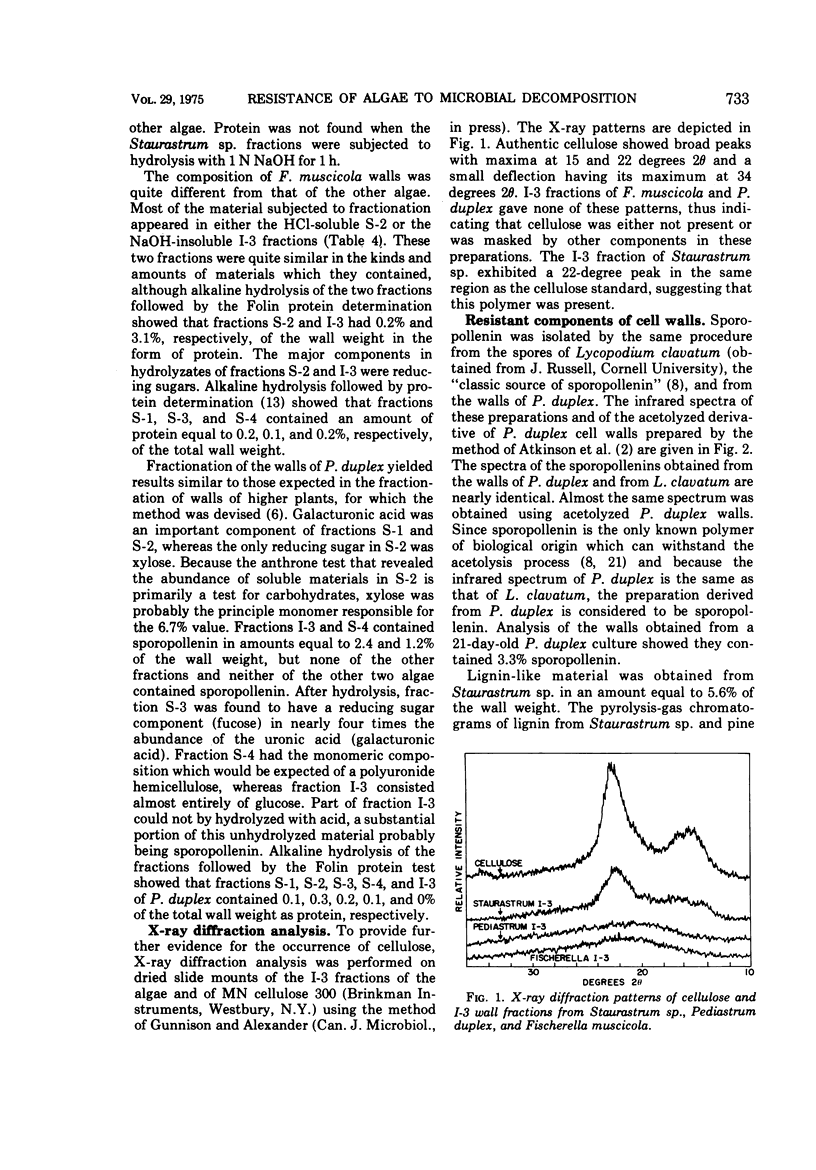
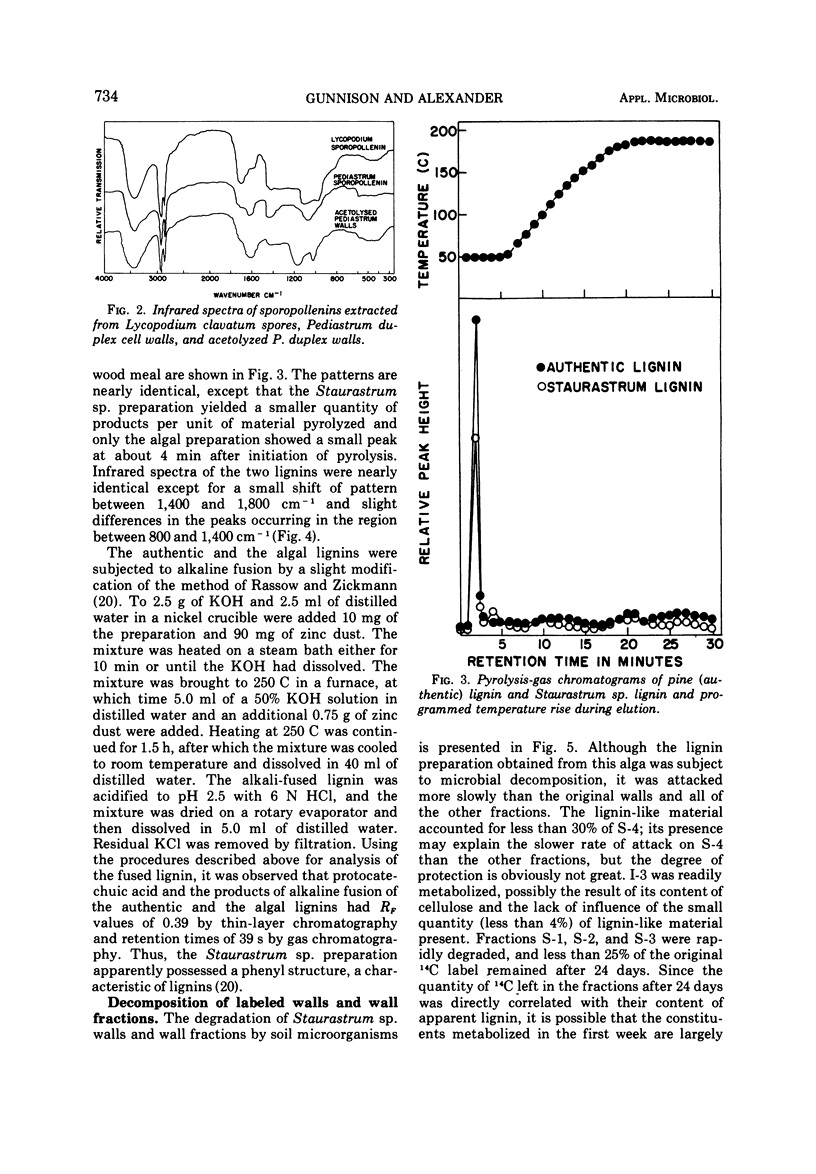
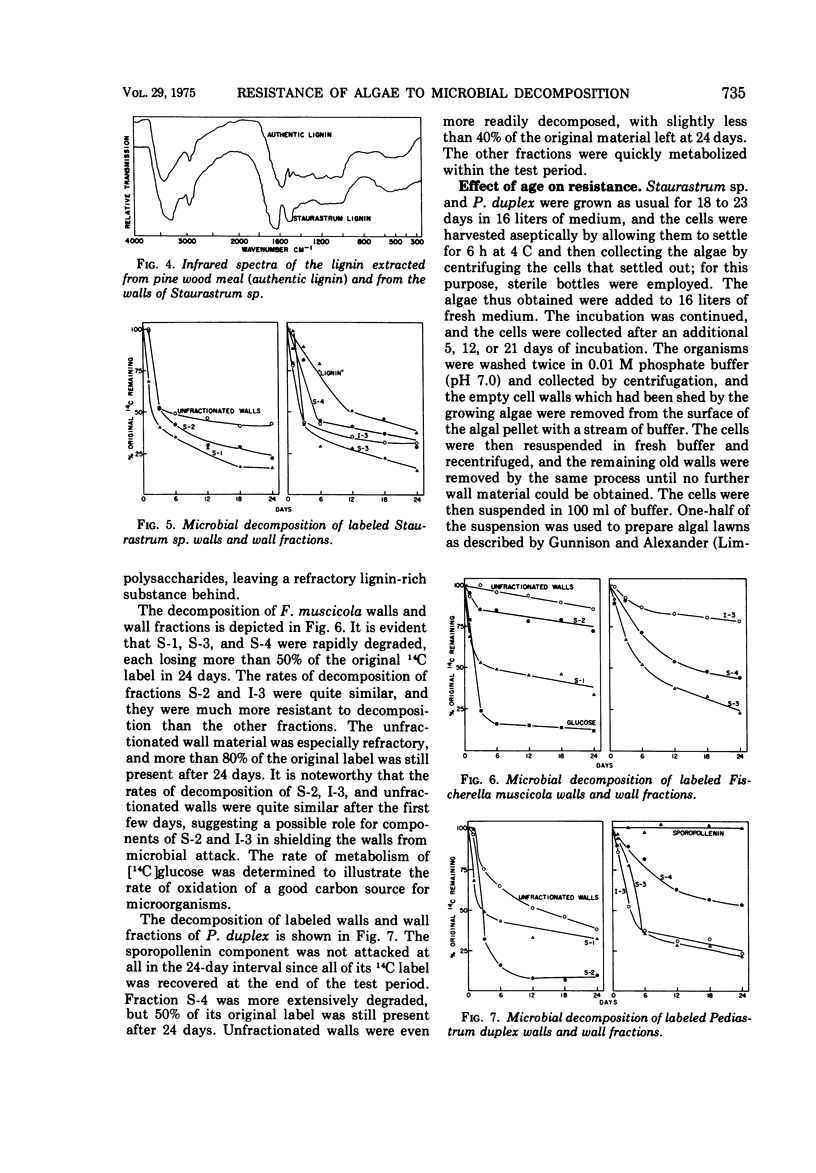
The basis for the resistance of certain algae to microbial decomposition in natural waters was investigated using Pediastrum duplex, Staurastrum sp., and Fischerella muscicola as test organisms. Enzyme preparations previously found to convert susceptible algae into spheroplasts had no such effect on the resistant species, although glucose and galacturonic acid were released from P. duplex walls. Little protein or lipid but considerable carbohydrate was found in the walls of the refractory organisms, but resistance was not correlated with the presence of a unique sugar monomer. A substance present in Staurastrum sp. walls was characterized as lignin or lignin-like on the basis of its extraction characteristics, infrared spectrum, pyrolysis pattern, and content of an aromatic building block. Sporopollenin was found in P. duplex, and cellulose in Staurastrum sp. Cell walls of the algae were fractionated, and the fractions least susceptible to microbial degradation were the sporopollenin of P. duplex, the polyaromatic component of Staurastrum sp., and two F. muscicola fractions containing several sugar monomers. The sporopollenin content of P. duplex, the content of lignin or a related constituent of Staurastrum sp., and the resistance of the algae to microbial attack increased with age. It is suggested that resistance results from the presence of sporopollenin in P. duplex, a lignin-like material in Staurastrum sp., and possibly heteropolysaccharides in F. muscicola.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOROUGHS H., BONNER J. Effects of indoleacetic acid on metabolic pathways. Arch Biochem Biophys. 1953 Oct;46(2):279–290. doi: 10.1016/0003-9861(53)90201-x. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Alexander M. Resistance of Zygorhynchus species to lysis. J Bacteriol. 1971 Jun;106(3):938–945. doi: 10.1128/jb.106.3.938-945.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield B. J., Alexander M. Melanins and resistance of fungi to lysis. J Bacteriol. 1967 Apr;93(4):1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J., Shaw G. Relationship of kerogen and sporopollenin--a reply. Nature. 1970 Jul 11;227(5254):195–196. doi: 10.1038/227195a0. [DOI] [PubMed] [Google Scholar]
- Helling C. S., Bollag J. M. Microanalysis of catechols as lead salts by infrared spectroscopy. Anal Biochem. 1968 Jul;24(1):34–43. doi: 10.1016/0003-2697(68)90057-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millington W. F., Gawlik S. R. Silica in the wall of Pediastrum. Nature. 1967 Oct 7;216(5110):68–68. doi: 10.1038/216068a0. [DOI] [PubMed] [Google Scholar]


