Abstract
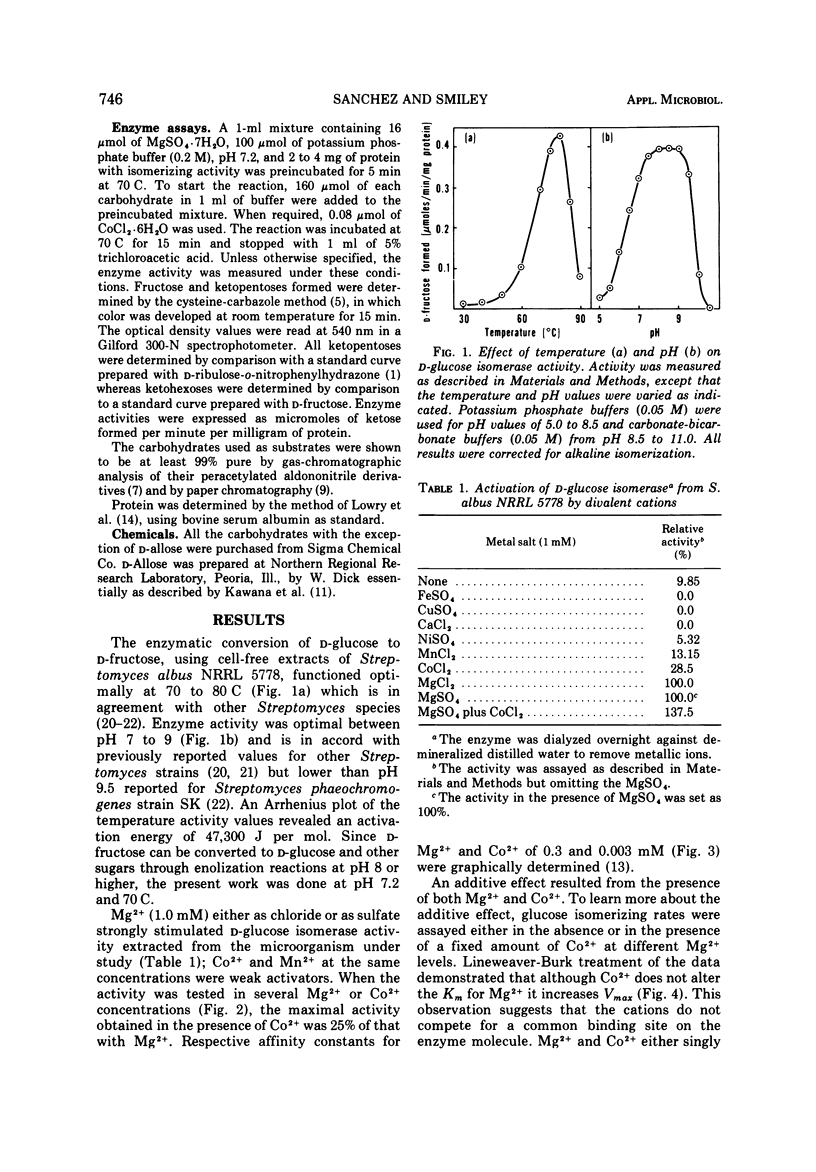
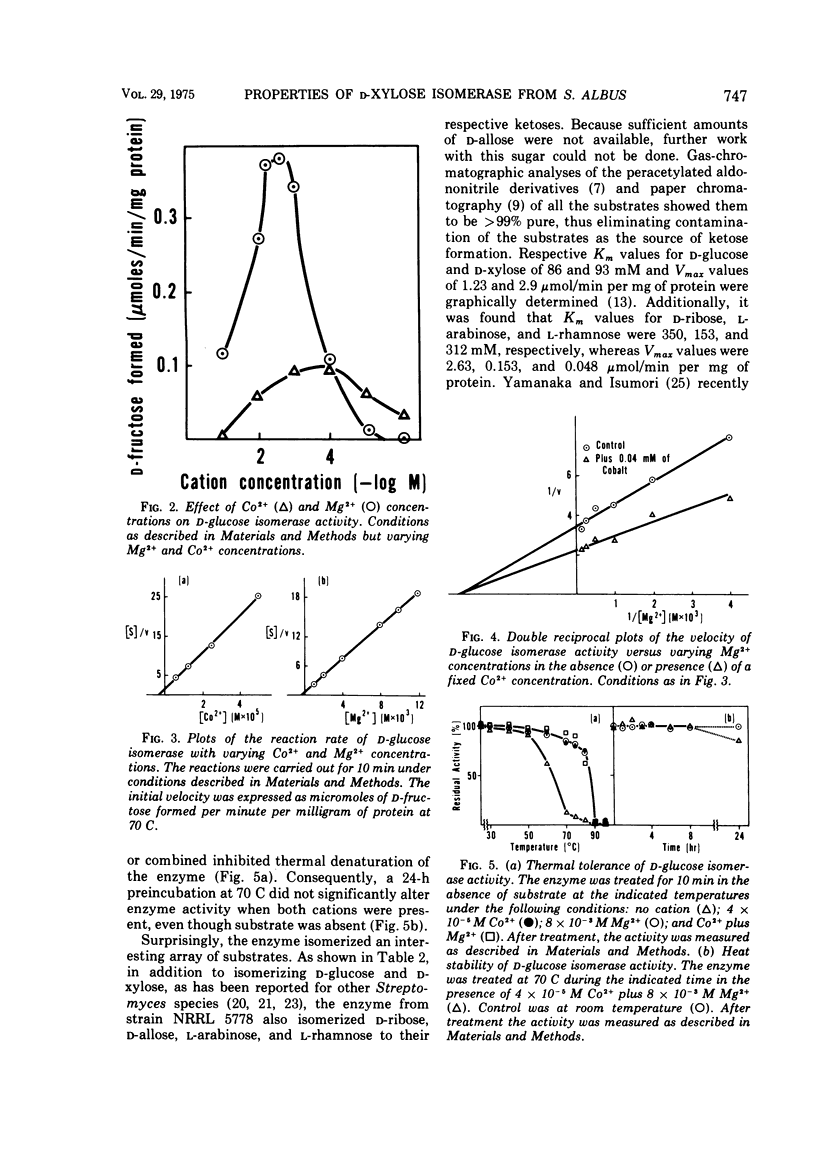
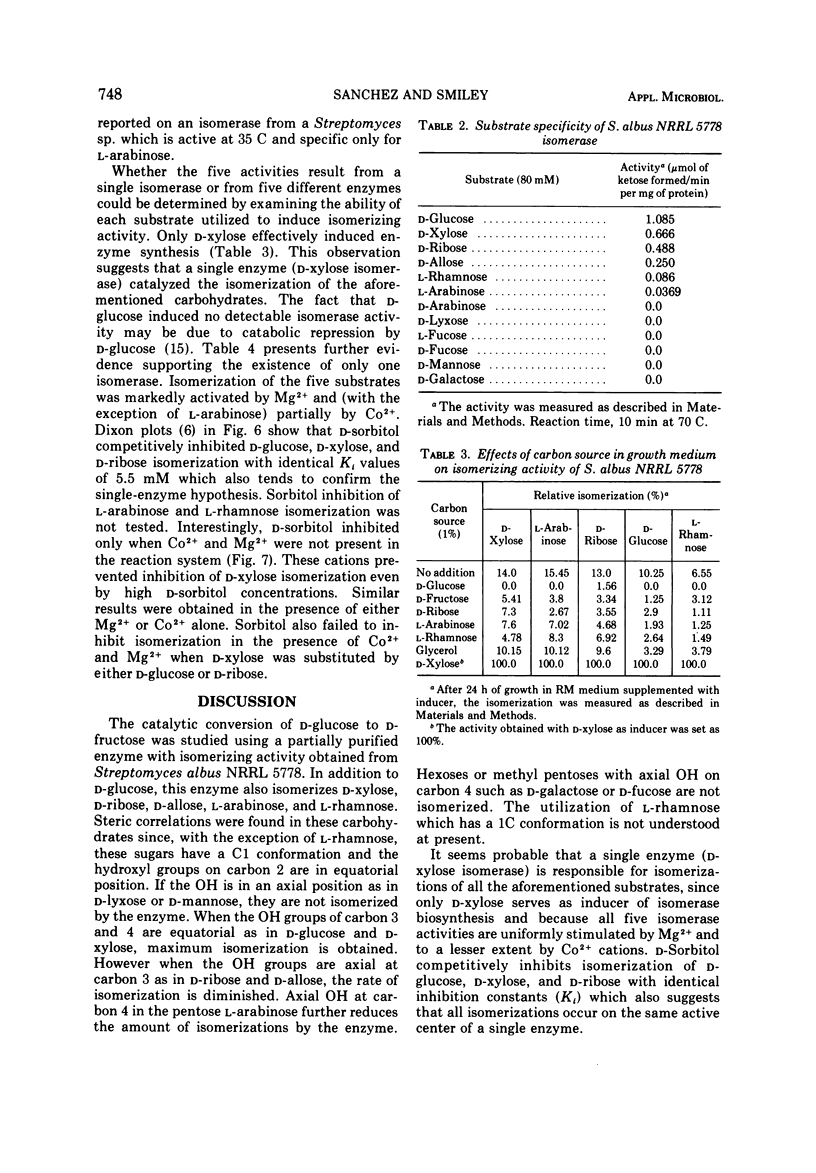
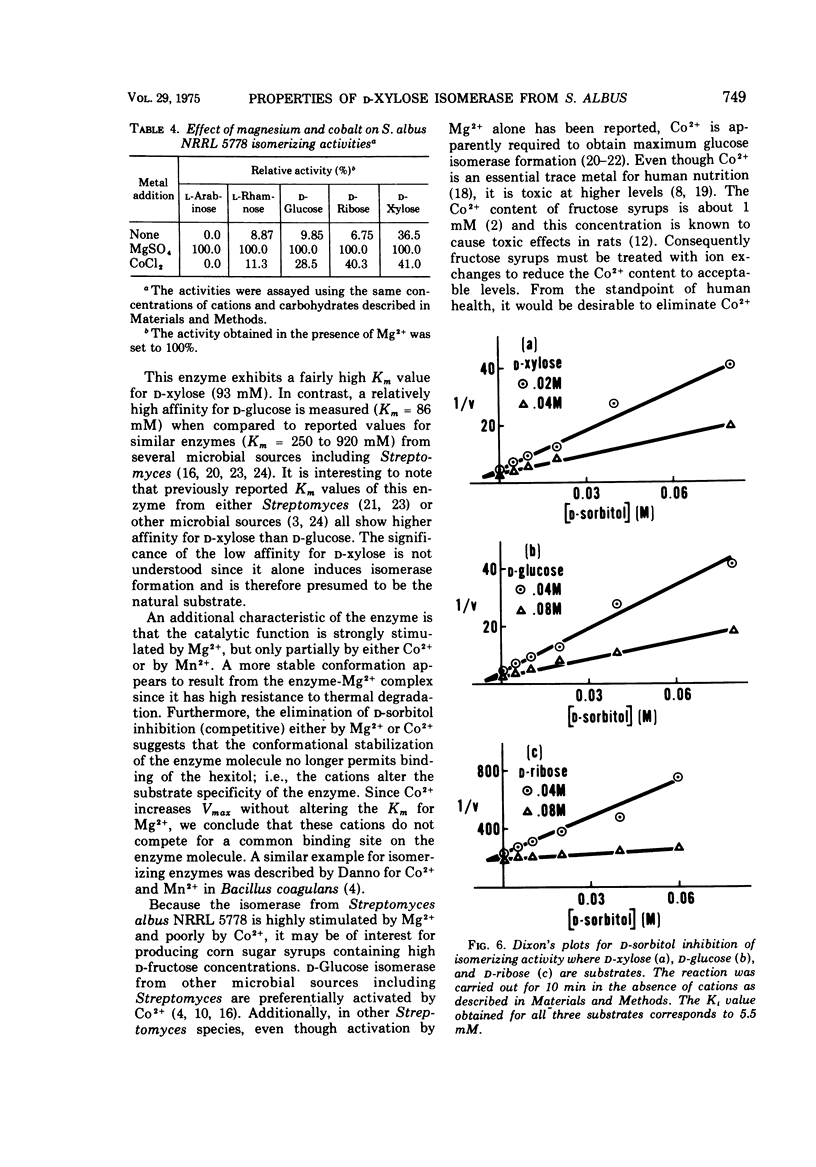
A partially purified D-xylose isomerase has been isolated from cells of Streptomyces albus NRRL 5778 and some of its properties have been determined. D-Glucose, D-xylose, D-ribose, L-arabinose, and L-rhamnose served as substrates for the enzyme with respective Km values of 86, 93, 350, 153, and 312 mM and Vmax values measuring 1.23, 2.9, 2.63, 0.153, and 0.048 μmol/min per mg of protein. The hexose D-allose was also isomerized. The enzyme was strongly activated by 1.0 mM Mg2+ but only partially activated by 1.0 mM Co2+. The respective Km values for Mg2+ and Co2+ were 0.3 and 0.003 mM. Mg2+ and Co2+ appear to have separate binding sites on the isomerase. These cations also protect the enzyme from thermal denaturation and from D-sorbitol inhibition. The optimum temperature for ketose formation was 70 to 80 C at pH values ranging from 7 to 9. D-Sorbitol acts as a competitive inhibitor with a Ki of 5.5 mM against D-glucose, D-xylose, and D-ribose. Induction experiments, Mg2+ activation, and D-sorbitol D-sorbitol inhibition indicated that a single enzyme (D-xylose isomerase) was responsible for the isomerization of the pentoses, methyl pentose, and glucose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBZINER H., RAYBIN H. W. Poison control...accidental cobalt poisoning. Arch Pediatr. 1961 May;78:200–205. [PubMed] [Google Scholar]
- LEVY H., LEVISON V., SCHADE A. L. The effect of cobalt on the activity of certain enzymes in homogenates of rat tissue. Arch Biochem. 1950 Jun;27(1):34–40. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MARSHALL R. O., KOOI E. R. Enzymatic conversion of D-glucose to D-fructose. Science. 1957 Apr 5;125(3249):648–649. doi: 10.1126/science.125.3249.648. [DOI] [PubMed] [Google Scholar]
- Schroeder H. A., Nason A. P., Tipton I. H. Essential trace metals in man: cobalt. J Chronic Dis. 1967 Nov-Dec;20(11):869–890. doi: 10.1016/0021-9681(67)90024-0. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Smiley K. L. Free and immobilized glucose isomerase from Streptomyces phaeochromogenes. Appl Microbiol. 1971 Apr;21(4):588–593. doi: 10.1128/am.21.4.588-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K. Purification, crystallization and properties of the D-xylose isomerase from Lactobacillus brevis. Biochim Biophys Acta. 1968 Mar 25;151(3):670–680. doi: 10.1016/0005-2744(68)90015-6. [DOI] [PubMed] [Google Scholar]



