Abstract
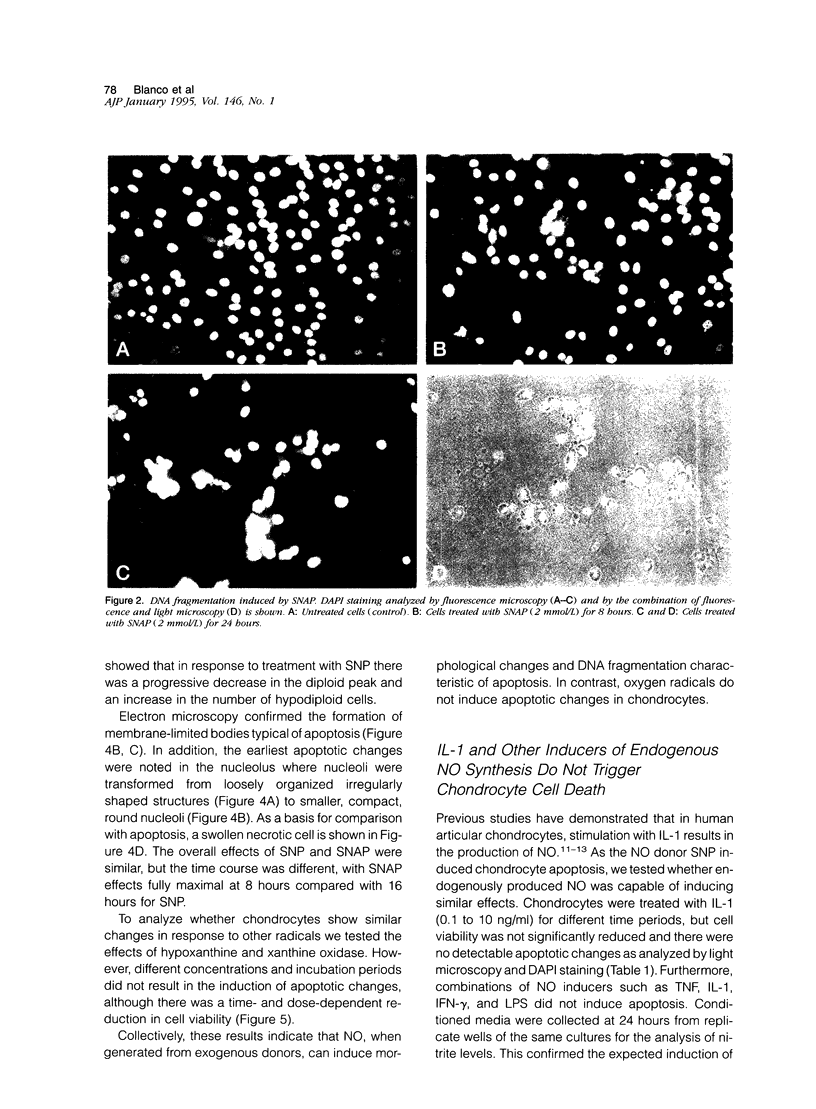
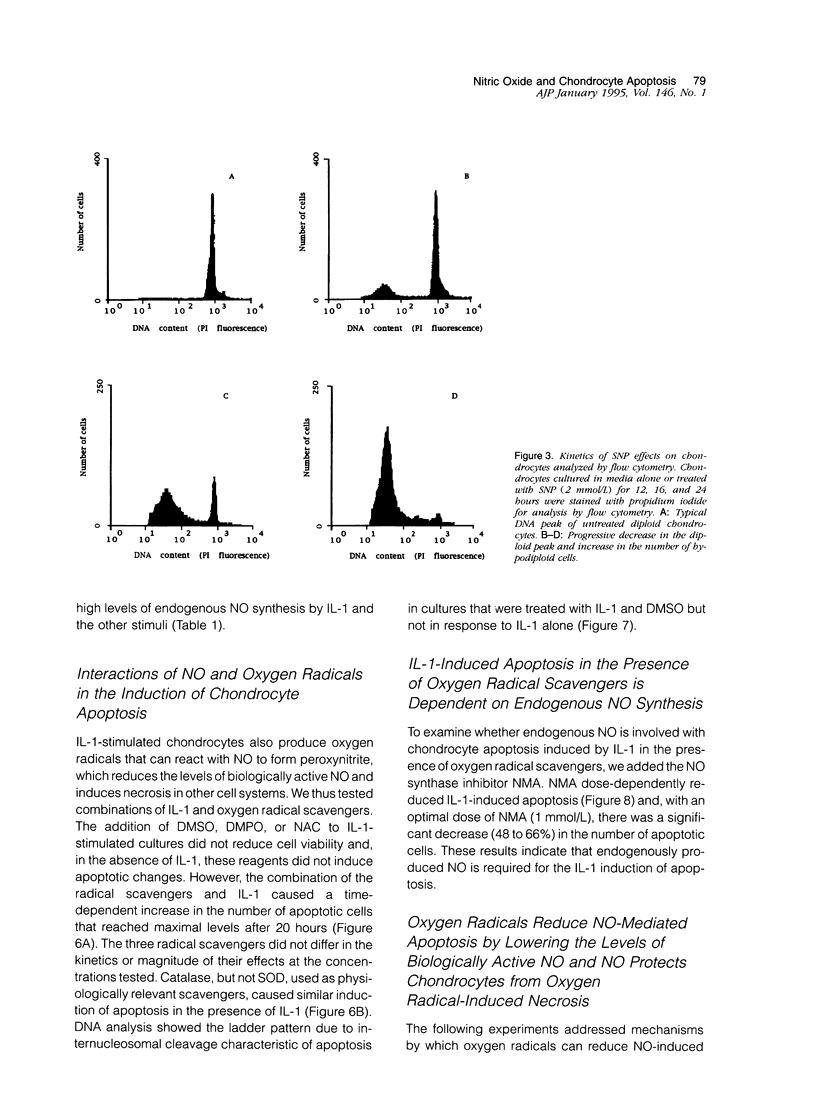
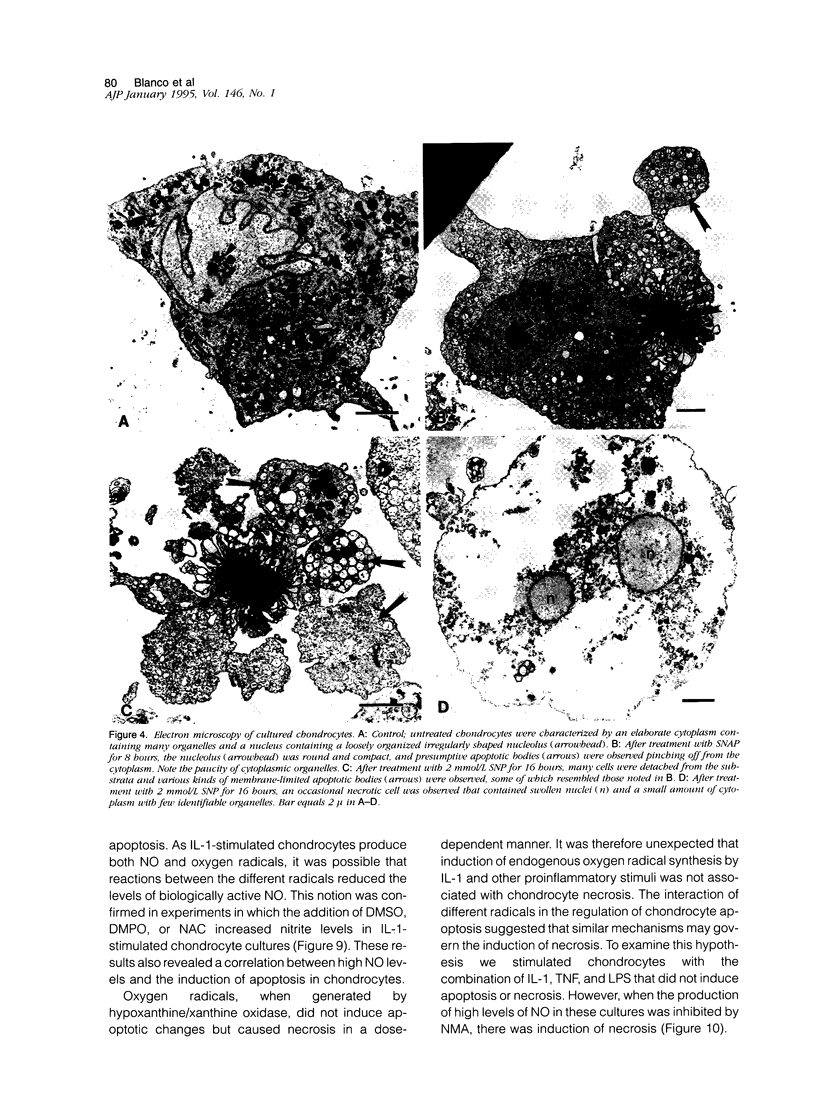
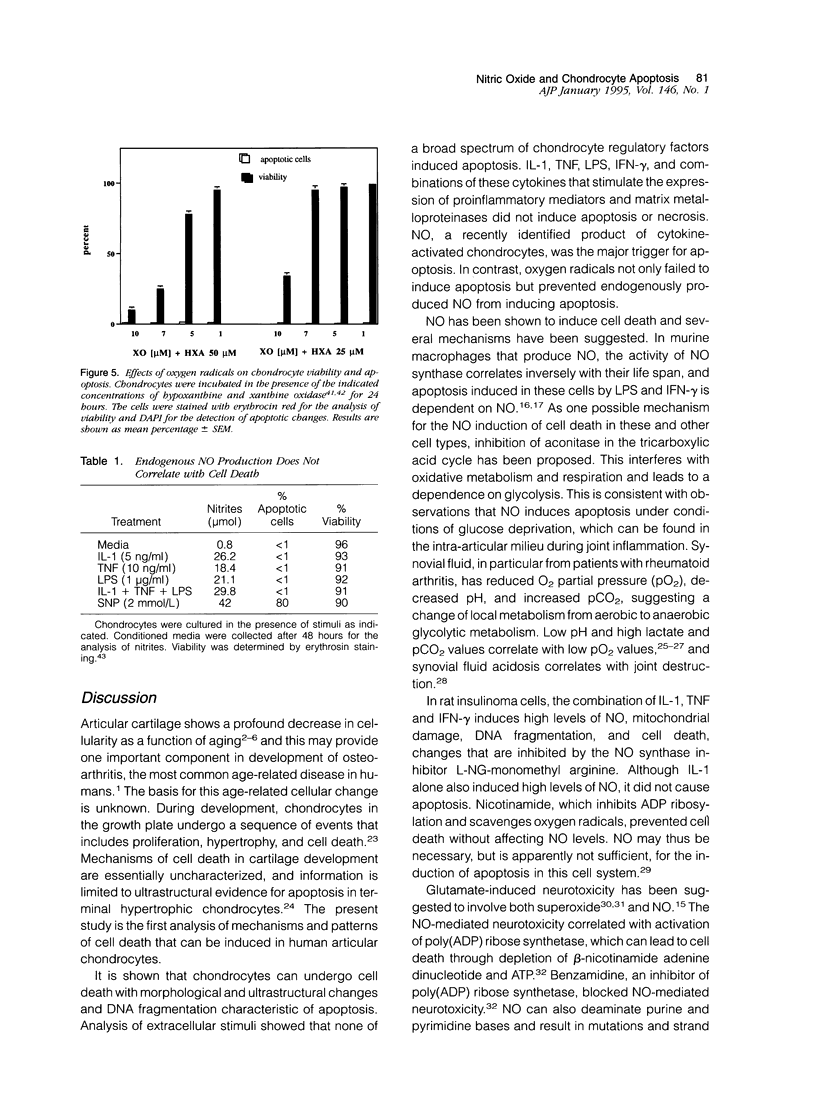
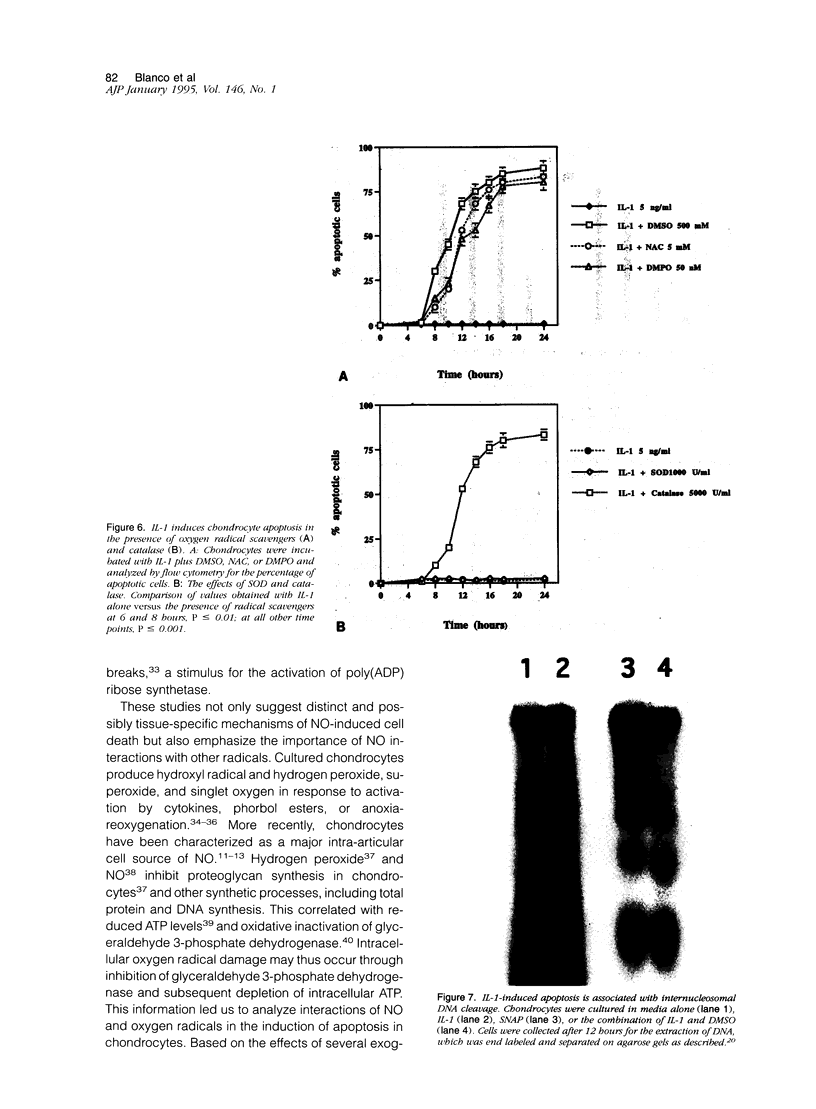
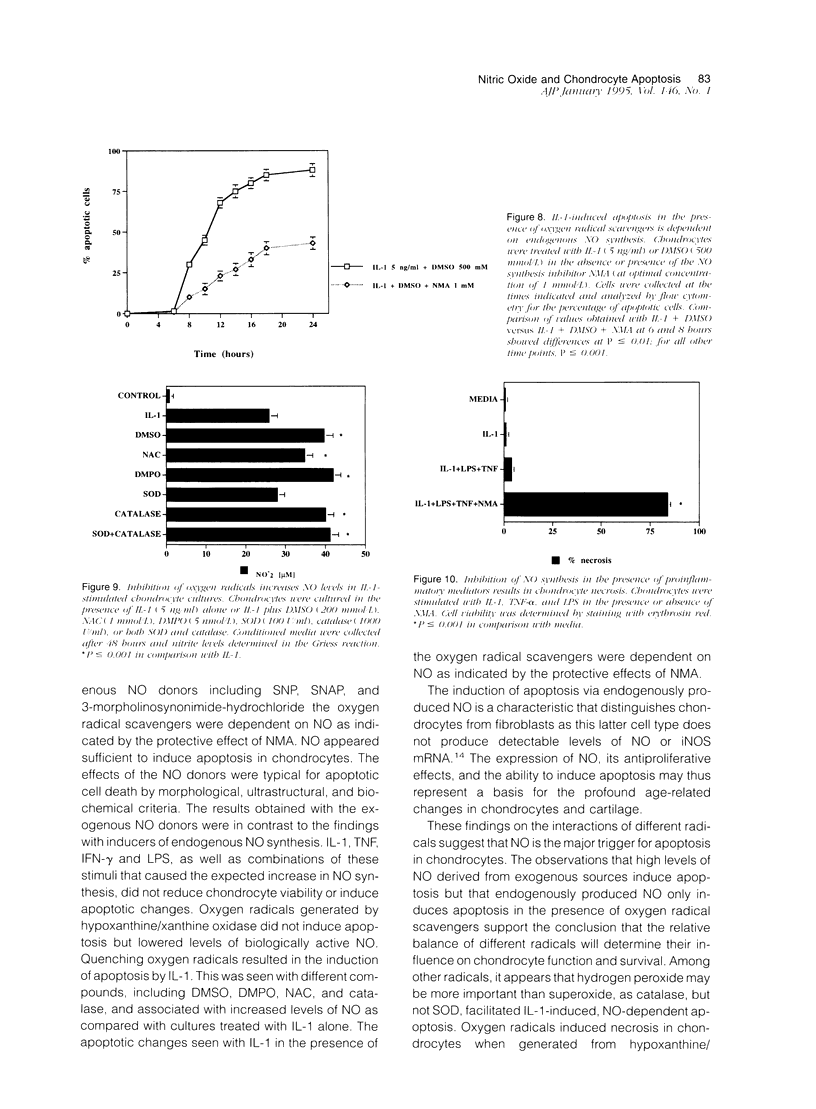
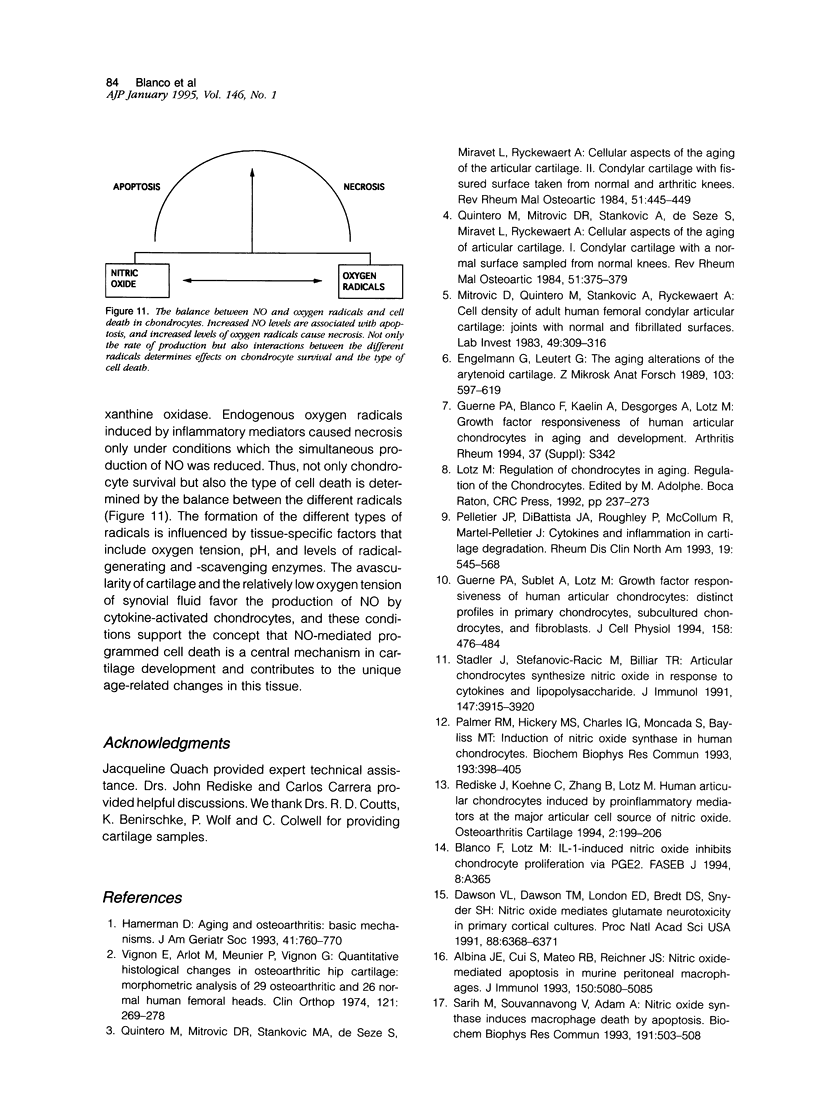
Chondrocytes stimulated with IL-1 produce high levels of nitric oxide (NO), which inhibits proliferation induced by transforming growth factor-beta or serum. This study analyzes the role of NO and IL-1 in the induction of chondrocyte cell death. NO generated from sodium nitroprusside induced apoptosis in cultured chondrocytes as demonstrated by electron microscopy, 4',6-dianidino-2-phenylindole dihydrochloride staining, FACS analysis, and histochemical detection of DNA fragmentation. Similar results were obtained with two other NO donors, 3-morpholinosynonimide-hydrochloride and s-nitroso-N-acetyl-D-L-penicillamine. In contrast, oxygen radicals generated by hypoxanthine/xanthine oxidase caused necrosis but did not induce chondrocyte apoptosis. To analyze whether endogenously generated NO induces apoptosis, chondrocytes were stimulated with IL-1, but there was no evidence for apoptotic changes. Combinations of NO inducers such as IL-1, lipopolysaccharide, tumor necrosis factor, and interferon-gamma also failed to trigger apoptosis. IL-1-stimulated chondrocytes are known to produce oxygen radicals that react with NO to form products that can induce cell death in other systems. We thus tested IL-1 in combination with the oxygen radical scavengers N-acetyl cysteine, dimethyl sulfoxide, or 5,5'-dimetylpyrroline 1-oxide. Under these conditions IL-1 was able to induce apoptosis, which was inhibited in a dose-dependent manner by the NO synthase inhibitor N-monomethyl L-arginine. Conversely, endogenous oxygen radicals induced by inflammatory mediators caused necrosis under conditions in which the simultaneous production of NO was reduced. These results suggest that NO, but not oxygen radicals, is the primary inducer of apoptosis in human articular chondrocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Cui S., Mateo R. B., Reichner J. S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993 Jun 1;150(11):5080–5085. [PubMed] [Google Scholar]
- Baker M. S., Feigan J., Lowther D. A. Chondrocyte antioxidant defences: the roles of catalase and glutathione peroxidase in protection against H2O2 dependent inhibition of proteoglycan biosynthesis. J Rheumatol. 1988 Apr;15(4):670–677. [PubMed] [Google Scholar]
- Baker M. S., Feigan J., Lowther D. A. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J Rheumatol. 1989 Jan;16(1):7–14. [PubMed] [Google Scholar]
- Burkart V., Imai Y., Kallmann B., Kolb H. Cyclosporin A protects pancreatic islet cells from nitric oxide-dependent macrophage cytotoxicity. FEBS Lett. 1992 Nov 16;313(1):56–58. doi: 10.1016/0014-5793(92)81183-m. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann G., Leutert G. Zu Alternsveränderungen der Cartilago arytaenoidea. Z Mikrosk Anat Forsch. 1989;103(4):597–619. [PubMed] [Google Scholar]
- Falchuk K. H., Goetzl E. J., Kulka J. P. Respiratory gases of synovial fluids. An approach to synovial tissue circulatory-metabolic imbalance in rheumatoid arthritis. Am J Med. 1970 Aug;49(2):223–231. doi: 10.1016/s0002-9343(70)80078-x. [DOI] [PubMed] [Google Scholar]
- Geborek P., Saxne T., Pettersson H., Wollheim F. A. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J Rheumatol. 1989 Apr;16(4):468–472. [PubMed] [Google Scholar]
- Guerne P. A., Sublet A., Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994 Mar;158(3):476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Aging and osteoarthritis: basic mechanisms. J Am Geriatr Soc. 1993 Jul;41(7):760–770. doi: 10.1111/j.1532-5415.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Deby-Dupont G., Deby C., De Bruyn M., Lamy M., Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993 Jul;32(7):562–567. doi: 10.1093/rheumatology/32.7.562. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Rassenti L. Z., Meisenholder G., Carson D. A., Kipps T. J. Autoantigen inhibits apoptosis of a human B cell leukemia that produces pathogenic rheumatoid factor. J Immunol. 1993 Dec 15;151(12):7273–7283. [PubMed] [Google Scholar]
- Krause A. W., Carley W. W., Webb W. W. Fluorescent erythrosin B is preferable to trypan blue as a vital exclusion dye for mammalian cells in monolayer culture. J Histochem Cytochem. 1984 Oct;32(10):1084–1090. doi: 10.1177/32.10.6090533. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M., Pietri S., Culcasi M., Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993 Aug 5;364(6437):535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lewinson D., Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat Rec. 1992 Aug;233(4):504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970 Nov-Dec;13(6):769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- Maier R., Ganu V., Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993 Oct 15;268(29):21527–21532. [PubMed] [Google Scholar]
- Mitrovic D., Quintero M., Stankovic A., Ryckewaert A. Cell density of adult human femoral condylar articular cartilage. Joints with normal and fibrillated surfaces. Lab Invest. 1983 Sep;49(3):309–316. [PubMed] [Google Scholar]
- Nguyen T., Brunson D., Crespi C. L., Penman B. W., Wishnok J. S., Tannenbaum S. R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Hickery M. S., Charles I. G., Moncada S., Bayliss M. T. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993 May 28;193(1):398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., DiBattista J. A., Roughley P., McCollum R., Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993 Aug;19(3):545–568. [PubMed] [Google Scholar]
- Quintero M., Mitrovic D. R., Stankovic A., de Sèze S., Miravet L., Ryckewaert A. Aspects cellulaires du vieillissement du cartilage articulaire. I. Cartilage condylien à surface normale, prèlevè dans les genoux normaux. Rev Rhum Mal Osteoartic. 1984 Jul-Sep;51(7-8):375–379. [PubMed] [Google Scholar]
- Quintero M., Mitrovic D. R., Stankovic M. A., de Sèze S., Miravet L., Ryckewaert A. Aspects cellulaires du vieillissement du cartilage articulaire. II. Cartilage condylien à surface fissurée prélevé dans les genoux "normaux" et "arthrosiques". Rev Rhum Mal Osteoartic. 1984 Oct;51(9):445–449. [PubMed] [Google Scholar]
- Rathakrishnan C., Tiku M. L. Lucigenin-dependent chemiluminescence in articular chondrocytes. Free Radic Biol Med. 1993 Aug;15(2):143–149. doi: 10.1016/0891-5849(93)90053-w. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Rösl F. A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 1992 Oct 11;20(19):5243–5243. doi: 10.1093/nar/20.19.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarih M., Souvannavong V., Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun. 1993 Mar 15;191(2):503–508. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Georgescu H. I., Evans C. H. Nitric oxide and energy production in articular chondrocytes. J Cell Physiol. 1994 May;159(2):274–280. doi: 10.1002/jcp.1041590211. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon W. L., Strynadka K., Schulz R., Rabinovitch A. Mechanisms of cytokine-induced destruction of rat insulinoma cells: the role of nitric oxide. Endocrinology. 1994 Mar;134(3):1006–1010. doi: 10.1210/endo.134.3.8119136. [DOI] [PubMed] [Google Scholar]
- Taskiran D., Stefanovic-Racic M., Georgescu H., Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994 Apr 15;200(1):142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- Tiku M. L., Liesch J. B., Robertson F. M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990 Jul 15;145(2):690–696. [PubMed] [Google Scholar]
- Treuhaft P. S., MCCarty D. J. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971 Jul-Aug;14(4):475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]
- Verma R. S., Conte R. A., Luke S., Sindwani V., Macera M. J. Deciphering the fluorescent variability of human genomic heterochromatin by DA/DAPI technique. Clin Genet. 1992 Nov;42(5):267–270. doi: 10.1111/j.1399-0004.1992.tb03253.x. [DOI] [PubMed] [Google Scholar]
- Vignon E., Arlot M., Meunier P., Vignon G. Quantitative histological changes in osteoarthritic hip cartilage. Morphometric analysis of 29 osteoarthritic and 26 normal human femoral heads. Clin Orthop Relat Res. 1974;(103):269–278. [PubMed] [Google Scholar]
- Vincent F., Corral M., Defer N., Adolphe M. Effects of oxygen free radicals on articular chondrocytes in culture: c-myc and c-Ha-ras messenger RNAs and proliferation kinetics. Exp Cell Res. 1991 Feb;192(2):333–339. doi: 10.1016/0014-4827(91)90049-z. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]