Abstract
The present study explored the role of murine monocyte chemotactic protein (MCP) in the T cell-mediated hypersensitive granulomatous response to Schistosoma mansoni eggs. The study examined the time course of local production, contribution to cellular infiltration, and the role of T cells in endogenous regulation. Synchronized pulmonary granulomas were induced under conditions of primary and secondary states of immunity. Primer-directed polymerase chain reaction analysis showed increased MCP mRNA expression in granulomatous lungs, mainly in the secondary response. Levels of MCP were measured by enzyme-linked immunosorbent assay in cultures of intact granulomas. Spontaneous MCP production was modest in primary granuloma cultures, reaching a maximum of 5.7 +/- 0.9 ng/ml by 16 days. In contrast, the secondary response showed augmented and accelerated production, achieving 13 +/- 2.0 ng/ml by 2 days. Immunohistochemical staining revealed the strongest MCP expression within microvascular adventitial cells or pericytes as well as in scattered mononuclear cells associated with granulomas. Staining was not detected in normal lungs. Passive immunization with anti-MCP-1 antibodies caused a 40% reduction in the secondary granuloma area but did not significantly affect the primary response. With adoptive cell transfer and T cell subset depletion, it was shown that Thy-1+ and CD5+ cells augmented, whereas CD8+ cells appeared to impair, MCP production. This provides direct evidence that MCP is involved in secondary Th2-mediated response to schistosome eggs and is subject to regulation by T cells.
Full text
PDF


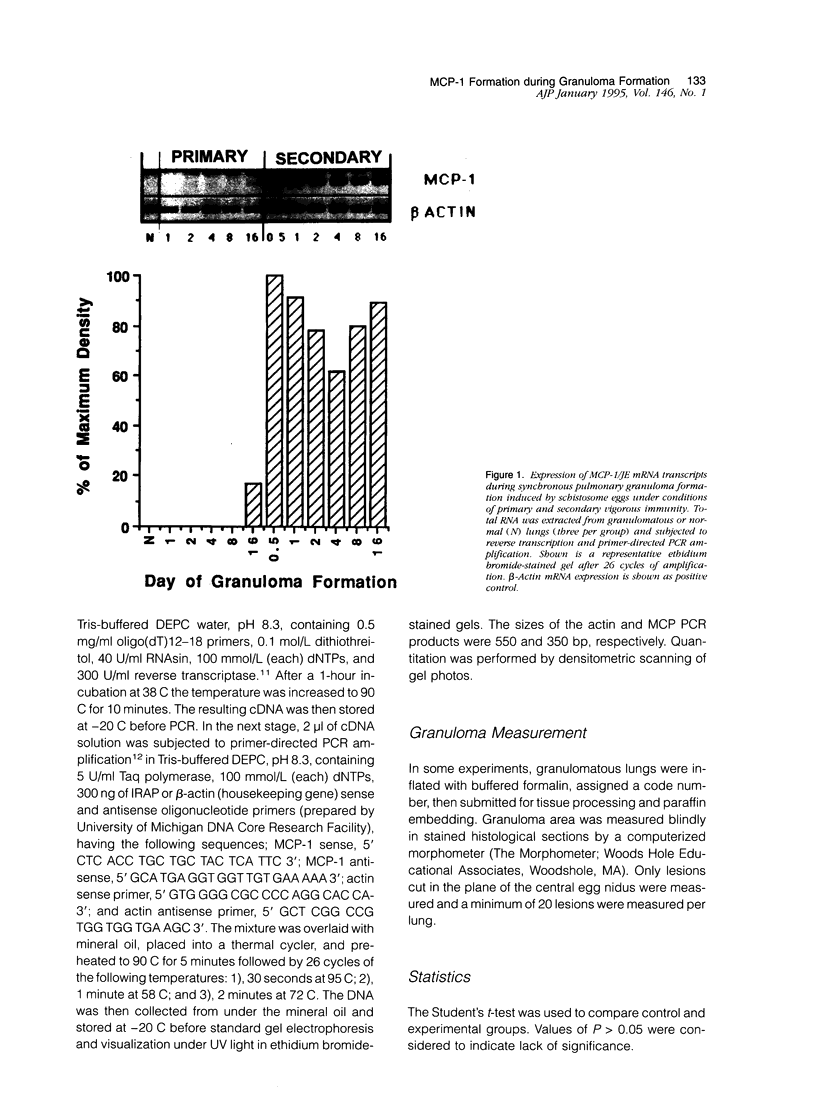
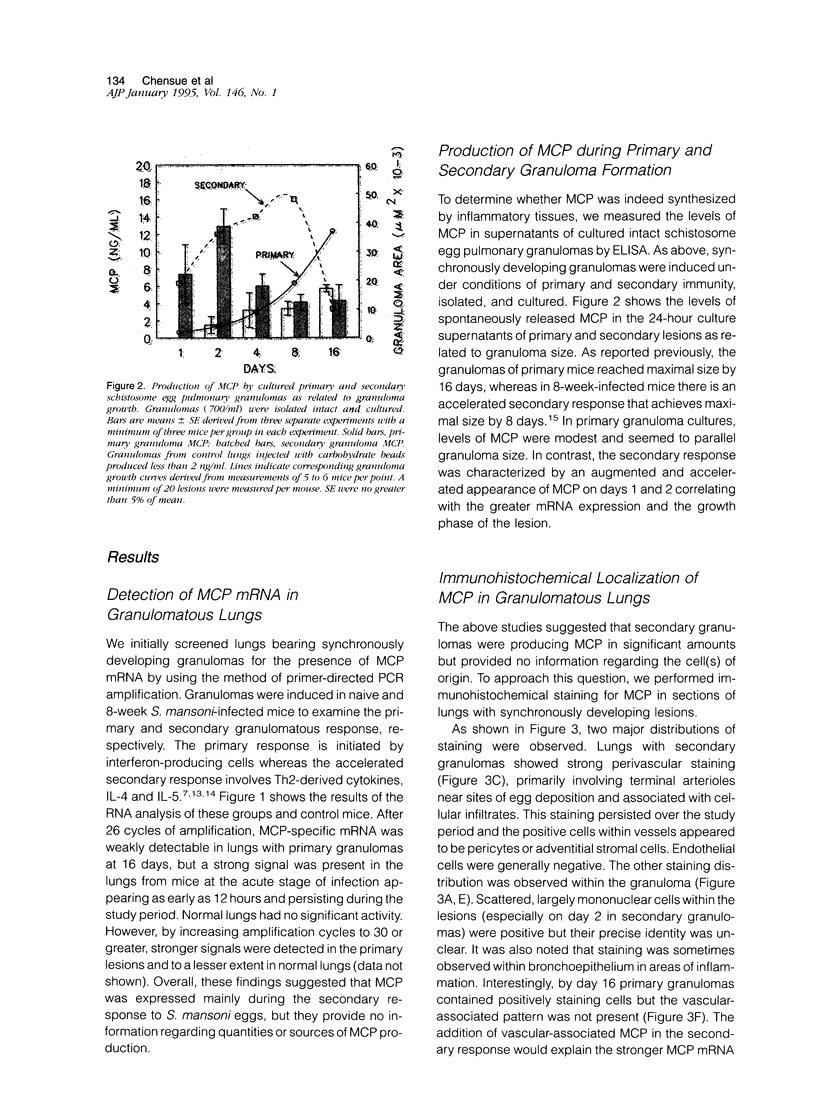
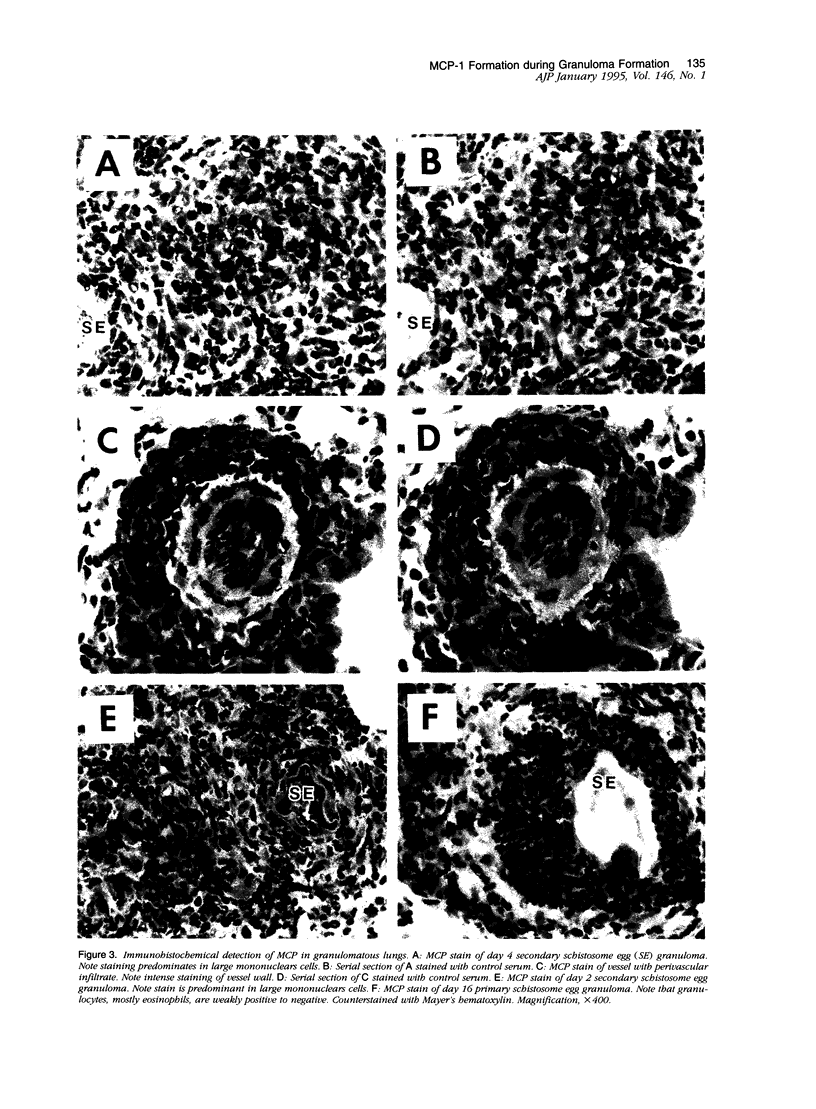



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam R., Lett-Brown M. A., Forsythe P. A., Anderson-Walters D. J., Kenamore C., Kormos C., Grant J. A. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for basophils. J Clin Invest. 1992 Mar;89(3):723–728. doi: 10.1172/JCI115648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Chensue S. W., Warmington K. S., Hershey S. D., Terebuh P. D., Othman M., Kunkel S. L. Evolving T cell responses in murine schistosomiasis. Th2 cells mediate secondary granulomatous hypersensitivity and are regulated by CD8+ T cells in vivo. J Immunol. 1993 Aug 1;151(3):1391–1400. [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Chensue S. W., Smith R. E., Strieter R. M., Warmington K., Wilke C., Kunkel S. L. Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha by inflammatory granuloma fibroblasts. Am J Pathol. 1994 Apr;144(4):711–718. [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Kunkel S. L., Strieter R. M., Warmington K., Chensue S. W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med. 1993 Jun 1;177(6):1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Pober J. S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991 Jun;138(6):1315–1319. [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Walz A., Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991 Aug 15;78(4):1112–1116. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Haskard D. O. IL-4 increases human endothelial cell adhesiveness for T cells but not for neutrophils. J Immunol. 1990 Apr 15;144(8):3060–3065. [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]