Abstract
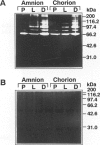
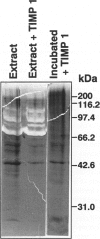
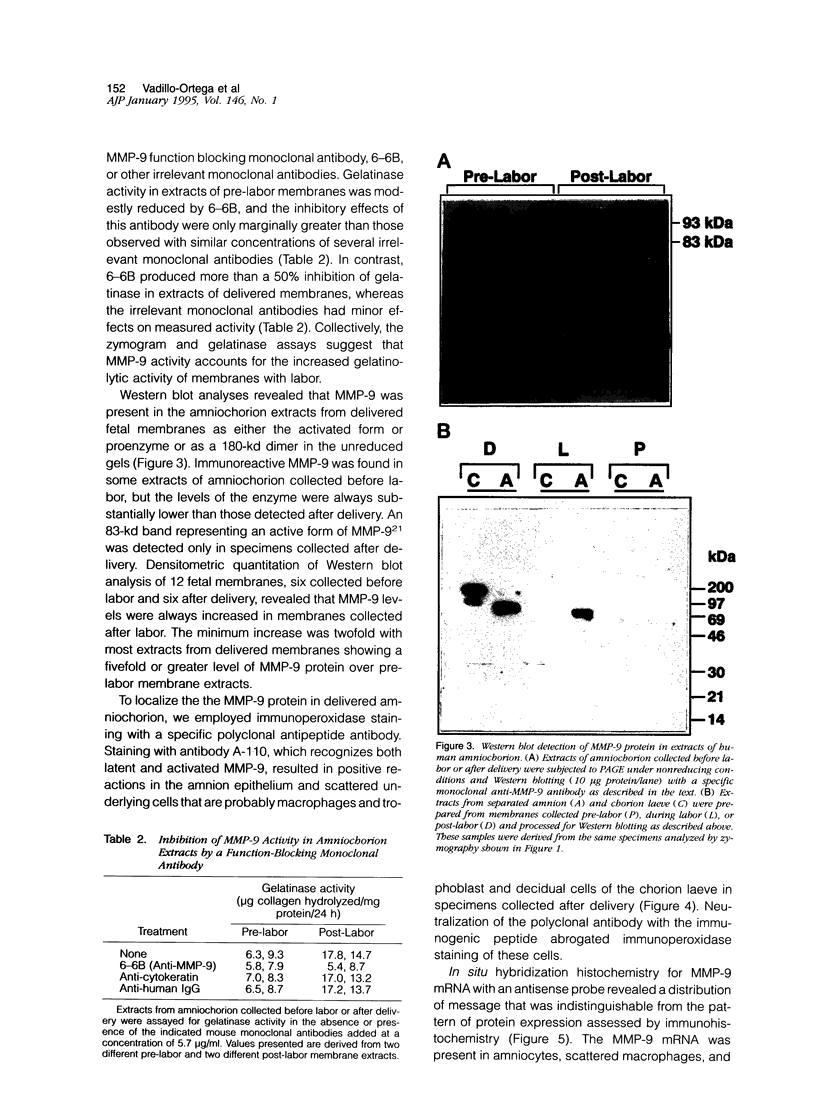
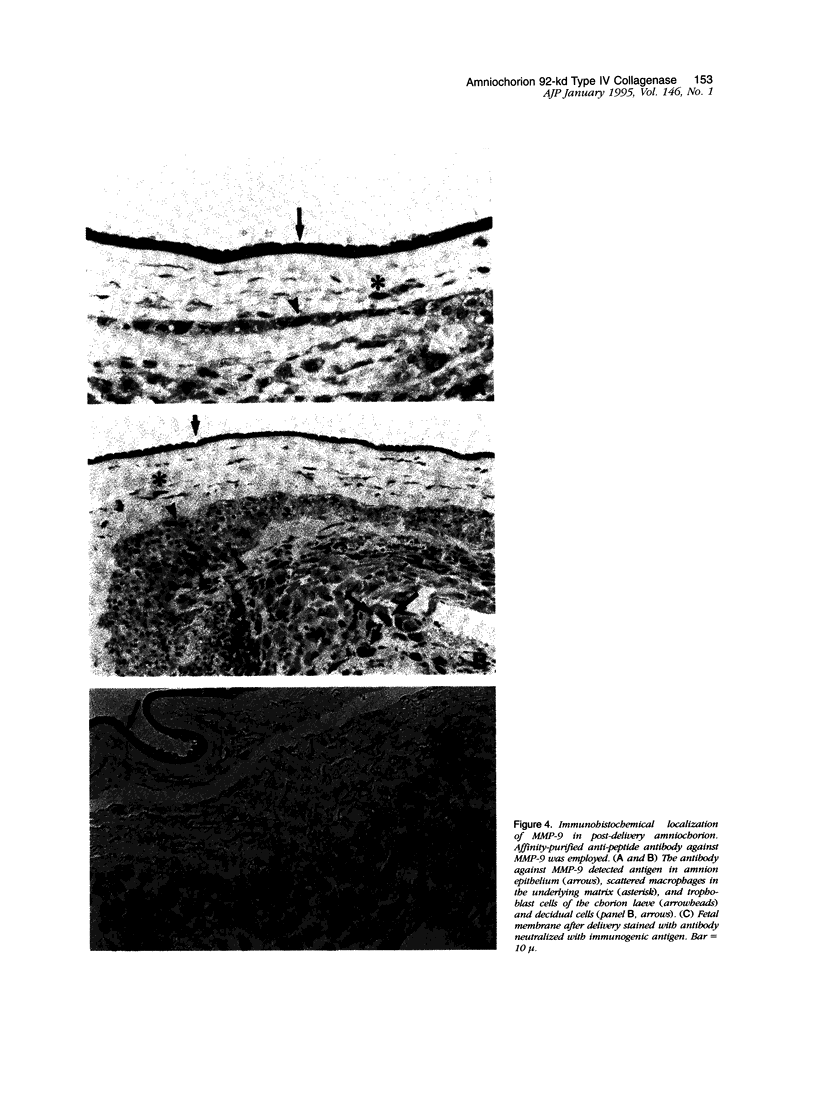
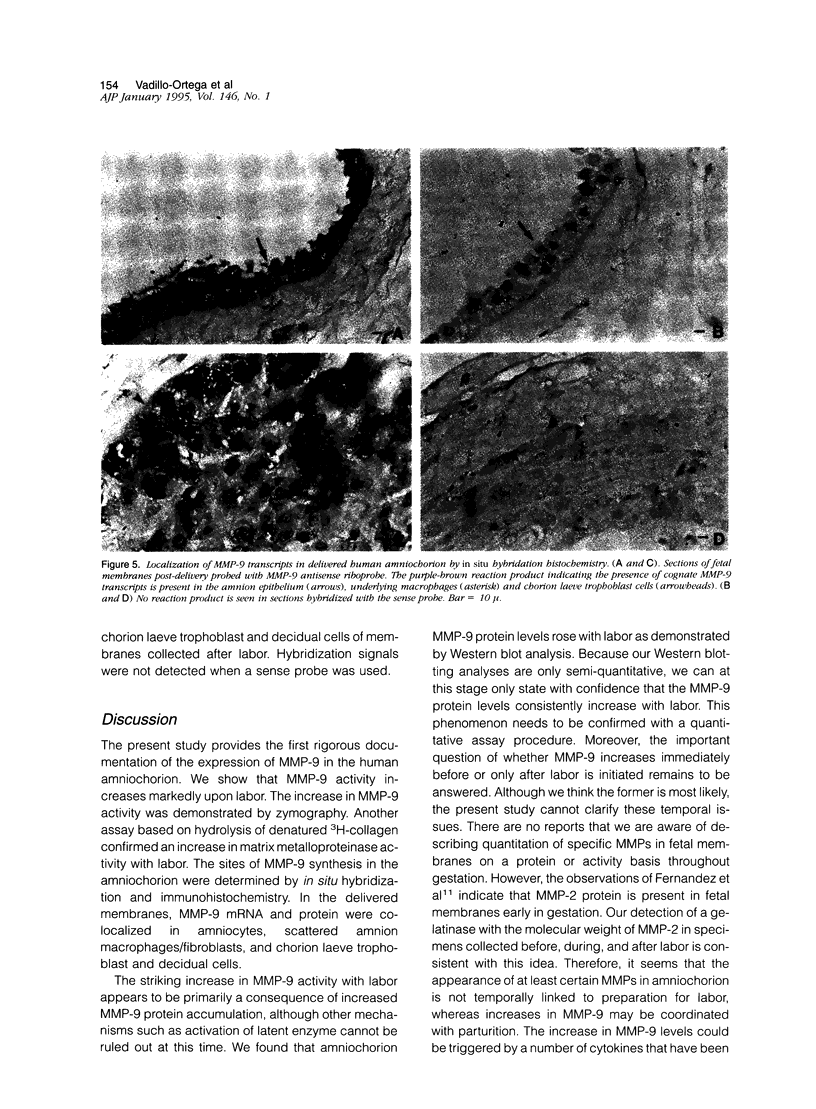
To determine whether specific collagenolytic enzymes are expressed in human fetal membranes with labor, we examined gelatinase activity in extracts of amniochorion by zymography. The 92-kd gelatinase (MMP-9) was barely detectable in extracts of fetal membranes before the onset of labor but was readily demonstrable in extracts prepared from membranes isolated from laboring women or membranes collected immediately after delivery. In contrast, the 72-kd gelatinase (MMP-2) was detectable in extracts from pre- and post-labor membranes. Ethylenediaminetetracetic acid and the tissue inhibitor of metalloproteinases, TIMP-1, inhibited the gelatinase activities detected by zymography, confirming that the enzymes are metalloproteinase. Assay of amniochorion gelatinase activity using a radiolabeled denatured collagen substrate revealed a more than twofold increase in activity comparing pre-labor with post-labor fetal membrane extracts. A function-blocking anti-MMP-9 monoclonal antibody inhibited pre-labor membrane gelatinase activity by approximately 11.5%, which was only slightly greater inhibition than observed with irrelevant monoclonal antibodies. However, post-labor membrane gelatinase activity was reduced by 53% by the function-blocking antibody, indicating that MMP-9 is a major contributor to the increased gelatinase activity extractable from post-labor membranes. Western blot analyses demonstrated increased MMP-9 protein in amniochorion extracts after onset of labor. MMP-9 protein and mRNA were co-localized in amnion epithelium, underlying macrophages and chorion laeve trophoblast and decidual cells after labor. We conclude that 1) MMP-9 activity and protein in human amniochorion increases with labor and 2) MMP-9 is expressed by amnion epithelium, macrophages and chorion laeve trophoblast and decidual cells. The increased expression of MMP-9 may result in degradation of the extracellular matrix of the fetal membranes and facilitate their rupture under both physiological and pathological conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Campbell S., Allen T. D. The extracellular matrix of human amniotic epithelium: ultrastructure, composition and deposition. J Cell Sci. 1985 Nov;79:119–136. doi: 10.1242/jcs.79.1.119. [DOI] [PubMed] [Google Scholar]
- Artal R., Burgeson R. E., Hobel C. J., Hollister D. An in vitro model for the study of enzymatically mediated biomechanical changes in the chorioamniotic membranes. Am J Obstet Gynecol. 1979 Mar 15;133(6):656–659. doi: 10.1016/0002-9378(79)90014-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canete-Soler R., Litzky L., Lubensky I., Muschel R. J. Localization of the 92 kd gelatinase mRNA in squamous cell and adenocarcinomas of the lung using in situ hybridization. Am J Pathol. 1994 Mar;144(3):518–527. [PMC free article] [PubMed] [Google Scholar]
- Corcoran M. L., Stetler-Stevenson W. G., Brown P. D., Wahl L. M. Interleukin 4 inhibition of prostaglandin E2 synthesis blocks interstitial collagenase and 92-kDa type IV collagenase/gelatinase production by human monocytes. J Biol Chem. 1992 Jan 5;267(1):515–519. [PubMed] [Google Scholar]
- Fernandez P. L., Merino M. J., Nogales F. F., Charonis A. S., Stetler-Stevenson W., Liotta L. Immunohistochemical profile of basement membrane proteins and 72 kilodalton type IV collagenase in the implantation placental site. An integrated view. Lab Invest. 1992 May;66(5):572–579. [PubMed] [Google Scholar]
- Halaburt J. T., Uldbjerg N., Helmig R., Ohlsson K. The concentration of collagen and the collagenolytic activity in the amnion and the chorion. Eur J Obstet Gynecol Reprod Biol. 1989 Apr;31(1):75–82. doi: 10.1016/0028-2243(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Himelstein B. P., Canete-Soler R., Bernhard E. J., Muschel R. J. Induction of fibroblast 92 kDa gelatinase/type IV collagenase expression by direct contact with metastatic tumor cells. J Cell Sci. 1994 Feb;107(Pt 2):477–486. doi: 10.1242/jcs.107.2.477. [DOI] [PubMed] [Google Scholar]
- Katsura M., Ito A., Hirakawa S., Mori Y. Human recombinant interleukin-1 alpha increases biosynthesis of collagenase and hyaluronic acid in cultured human chorionic cells. FEBS Lett. 1989 Feb 27;244(2):315–318. doi: 10.1016/0014-5793(89)80553-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGregor J. A., French J. I. Use of antibiotics for preterm premature rupture of membranes. Rationales and results. Obstet Gynecol Clin North Am. 1992 Jun;19(2):327–338. [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Ramos-DeSimone N., Moll U. M., Quigley J. P., French D. L. Inhibition of matrix metalloproteinase 9 activation by a specific monoclonal antibody. Hybridoma. 1993 Aug;12(4):349–363. doi: 10.1089/hyb.1993.12.349. [DOI] [PubMed] [Google Scholar]
- Skinner S. J., Campos G. A., Liggins G. C. Collagen content of human amniotic membranes: effect of gestation length and premature rupture. Obstet Gynecol. 1981 Apr;57(4):487–489. [PubMed] [Google Scholar]
- So T., Ito A., Sato T., Mori Y., Hirakawa S. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol Reprod. 1992 May;46(5):772–778. doi: 10.1095/biolreprod46.5.772. [DOI] [PubMed] [Google Scholar]
- Sunada H., Nagai Y. A rapid micro-assay method for gelatinolytic activity using tritium-labeled heat-denatured polymeric collagen as a substrate and its application to the detection of enzymes involved in collagen metabolism. J Biochem. 1980 Jun;87(6):1765–1771. doi: 10.1093/oxfordjournals.jbchem.a132921. [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F., González-Avila G., Karchmer S., Cruz N. M., Ayala-Ruiz A., Lama M. S. Collagen metabolism in premature rupture of amniotic membranes. Obstet Gynecol. 1990 Jan;75(1):84–88. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]
- Yurchenco P. D., Ruben G. C. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987 Dec;105(6 Pt 1):2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]