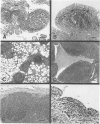
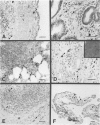
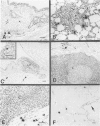
Abstract
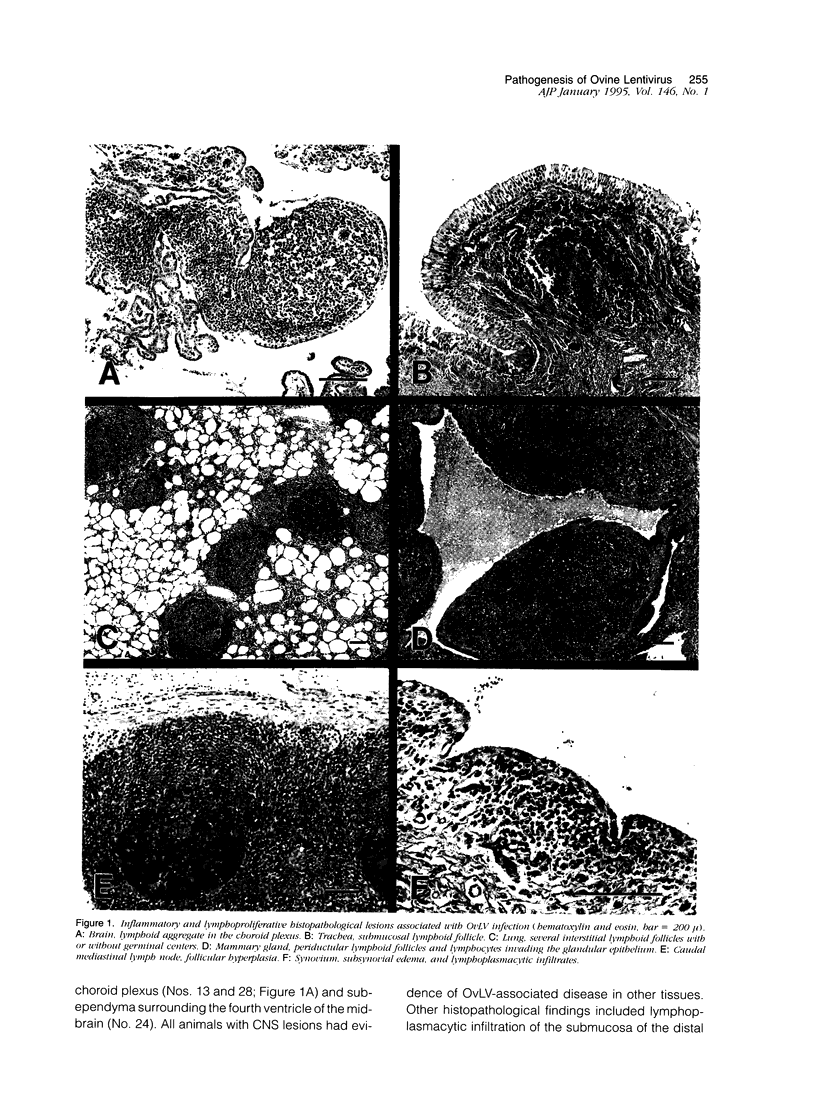
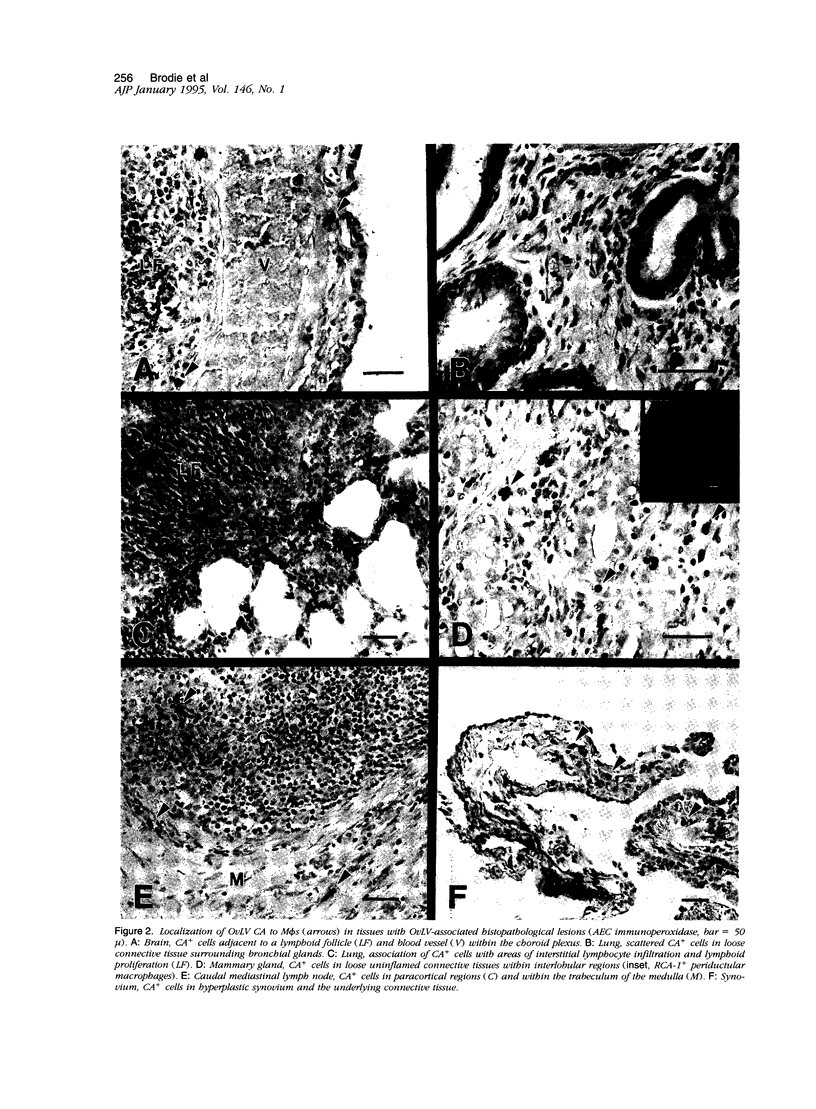
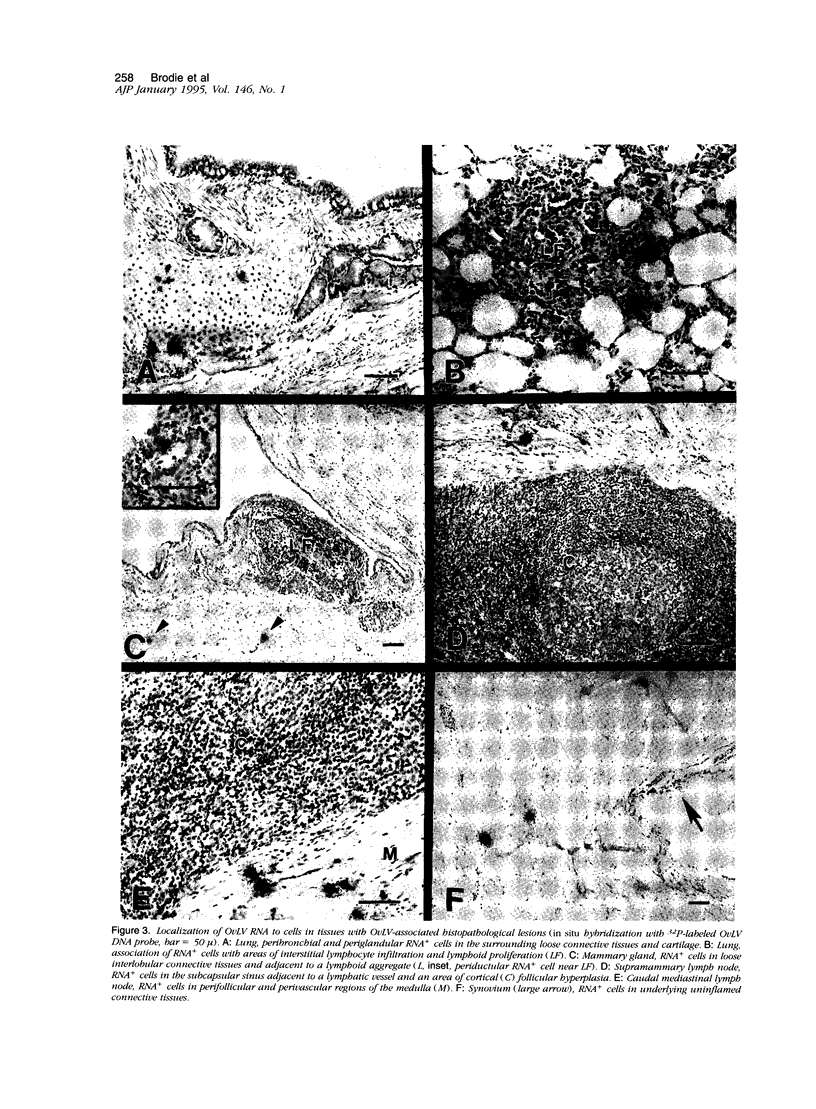
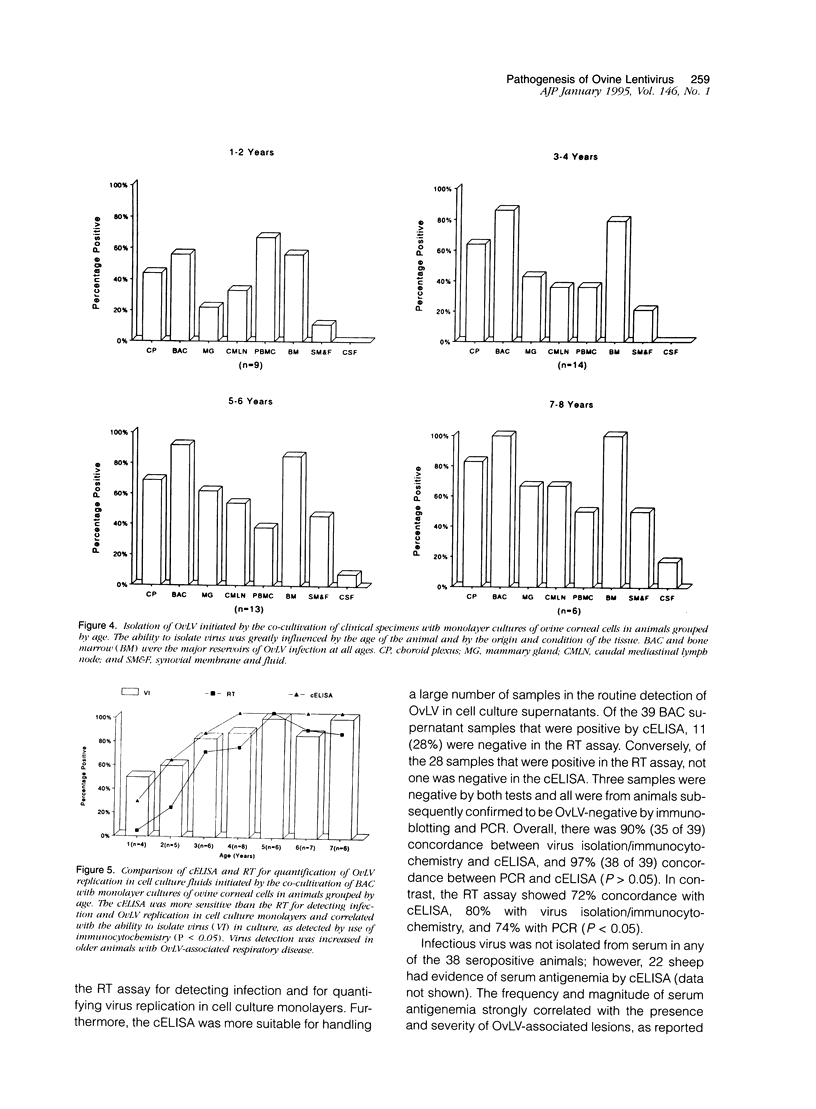
To better define the relationship between lentivirus infection and lymphoproliferative or inflammatory disease, we studied postmortem specimens of 38 sheep naturally infected with ovine lentivirus (OvLV) and with different clinical manifestations of OvLV-associated disease. Immunohistochemistry, in situ hybridization, and virus isolation were used to localize viral protein, viral RNA, and infectious virus to specific cells and tissues. Viral protein or infectious virus was found in cells morphologically and histochemically compatible with macrophages (M phi s), but only in lung, bone marrow, mammary gland, lymph node, spleen, synovium, brain, and spinal cord, frequently in association with lymphocyte infiltrates. In contrast, viral RNA was found in a variety of cell types, including epithelium, M phi s, and M phi-like cells, and in a wider range of tissues, with or without OvLV-associated lesions. In summary, these findings suggest that in vivo: 1), OvLV can enter a variety of cell types, 2), productive infection is restricted to cells of M phi lineage, and 3), cells expressing viral proteins are limited to specific tissues, those associated with OvLV-induced diseases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand R., Siegal F., Reed C., Cheung T., Forlenza S., Moore J. Non-cytocidal natural variants of human immunodeficiency virus isolated from AIDS patients with neurological disorders. Lancet. 1987 Aug 1;2(8553):234–238. doi: 10.1016/s0140-6736(87)90826-9. [DOI] [PubMed] [Google Scholar]
- Anderson B. C., Bulgin M. S., Adams S., Duelke B. Firm udder in periparturient ewes with lymphocytic accumulations, retrovirus infection, and milk unavailable at the teat. J Am Vet Med Assoc. 1985 Feb 15;186(4):391–393. [PubMed] [Google Scholar]
- Banks K. L., Jacobs C. A., Michaels F. H., Cheevers W. P. Lentivirus infection augments concurrent antigen-induced arthritis. Arthritis Rheum. 1987 Sep;30(9):1046–1053. doi: 10.1002/art.1780300912. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Braun M. J., Clements J. E., Gonda M. A. The visna virus genome: evidence for a hypervariable site in the env gene and sequence homology among lentivirus envelope proteins. J Virol. 1987 Dec;61(12):4046–4054. doi: 10.1128/jvi.61.12.4046-4054.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie S. J., Marcom K. A., Pearson L. D., Anderson B. C., de la Concha-Bermejillo A., Ellis J. A., DeMartini J. C. Effects of virus load in the pathogenesis of lentivirus-induced lymphoid interstitial pneumonia. J Infect Dis. 1992 Sep;166(3):531–541. doi: 10.1093/infdis/166.3.531. [DOI] [PubMed] [Google Scholar]
- Brodie S. J., Pearson L. D., Snowder G. D., DeMartini J. C. Host-virus interaction as defined by amplification of viral DNA and serology in lentivirus-infected sheep. Arch Virol. 1993;130(3-4):413–428. doi: 10.1007/BF01309670. [DOI] [PubMed] [Google Scholar]
- Brodie S. J., de la Concha-Bermejillo A., Koenig G., Snowder G. D., DeMartini J. C. Maternal factors associated with prenatal transmission of ovine lentivirus. J Infect Dis. 1994 Mar;169(3):653–657. doi: 10.1093/infdis/169.3.653. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro B. A., Cheng-Mayer C., Evans L. A., Levy J. A. HIV heterogeneity and viral pathogenesis. AIDS. 1988;2 (Suppl 1):S17–S27. doi: 10.1097/00002030-198800001-00004. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Brogden K. A., McClurkin A. W. Vasculitis associated with ovine progressive pneumonia virus infection in sheep. Am J Vet Res. 1985 Jan;46(1):61–64. [PubMed] [Google Scholar]
- DeMartini J. C., Brodie S. J., de la Concha-Bermejillo A., Ellis J. A., Lairmore M. D. Pathogenesis of lymphoid interstitial pneumonia in natural and experimental ovine lentivirus infection. Clin Infect Dis. 1993 Aug;17 (Suppl 1):S236–S242. doi: 10.1093/clinids/17.supplement_1.s236. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Hansen-Moosa A., Mori K., Bouvier D. P., King N. W., Daniel M. D., Ringler D. J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991 Jul;139(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Immunomorphologic and morphometric changes in pulmonary lymph nodes of sheep with progressive pneumonia. Vet Pathol. 1985 Jan;22(1):32–41. doi: 10.1177/030098588502200105. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Geballe A. P., Ventura P., Stowring L., Haase A. T. Quantitative analysis of visna virus replication in vivo. Virology. 1985 Feb;141(1):148–154. doi: 10.1016/0042-6822(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E., Clements J. E. A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods. 1985 Jun;11(2):93–103. doi: 10.1016/0166-0934(85)90033-3. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Clements J. E., Pezeshkpour G. H. Slow virus-macrophage interactions. Characterization of a transformed cell line of sheep alveolar macrophages that express a marker for susceptibility to ovine-caprine lentivirus infections. Lab Invest. 1984 Nov;51(5):547–555. [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgsson G., Houwers D. J., Pálsson P. A., Pétursson G. Expression of viral antigens in the central nervous system of visna-infected sheep: an immunohistochemical study on experimental visna induced by virus strains of increased neurovirulence. Acta Neuropathol. 1989;77(3):299–306. doi: 10.1007/BF00687582. [DOI] [PubMed] [Google Scholar]
- Gorrell M. D., Brandon M. R., Sheffer D., Adams R. J., Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992 May;66(5):2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Johnson L. K., Meyer A. L., Zink M. C. Detection of ovine lentivirus in seronegative sheep by in situ hybridization, PCR, and cocultivation with susceptible cells. Clin Immunol Immunopathol. 1992 Dec;65(3):254–260. doi: 10.1016/0090-1229(92)90155-h. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Kauffman S., Oleske J. M., Fikrig S., Denny T., Gadol C., Lee E. Polyclonal polymorphic B-cell lymphoproliferative disorder with prominent pulmonary involvement in children with acquired immune deficiency syndrome. Cancer. 1987 Apr 15;59(8):1455–1462. doi: 10.1002/1097-0142(19870415)59:8<1455::aid-cncr2820590811>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Zink C., Narayan O. Pathogenesis of ovine lentivirus-induced arthritis: phenotypic evaluation of T lymphocytes in synovial fluid, synovium, and peripheral circulation. Clin Immunol Immunopathol. 1989 Aug;52(2):323–330. doi: 10.1016/0090-1229(89)90183-9. [DOI] [PubMed] [Google Scholar]
- Kure K., Lyman W. D., Weidenheim K. M., Dickson D. W. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990 May;136(5):1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Akita G. Y., Russell H. I., DeMartini J. C. Replication and cytopathic effects of ovine lentivirus strains in alveolar macrophages correlate with in vivo pathogenicity. J Virol. 1987 Dec;61(12):4038–4042. doi: 10.1128/jvi.61.12.4038-4042.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Poulson J. M., Adducci T. A., DeMartini J. C. Lentivirus-induced lymphoproliferative disease. Comparative pathogenicity of phenotypically distinct ovine lentivirus strains. Am J Pathol. 1988 Jan;130(1):80–90. [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. C., Tsai S., Benish J. R., Shih J. W., Wear D. J., Wong D. M. Enhancement of HIV-1 cytocidal effects in CD4+ lymphocytes by the AIDS-associated mycoplasma. Science. 1991 Mar 1;251(4997):1074–1076. doi: 10.1126/science.1705362. [DOI] [PubMed] [Google Scholar]
- Marcom K. A., Brodie S. J., Pearson L. D., DeMartini J. C. Analysis of ovine lentivirus infectivity and replication by using a focal immunoassay and an antigen-capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1992 Nov;30(11):2852–2858. doi: 10.1128/jcm.30.11.2852-2858.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcom K. A., Pearson L. D., Chung C. S., Poulson J. M., DeMartini J. C. Epitope analysis of capsid and matrix proteins of North American ovine lentivirus field isolates. J Clin Microbiol. 1991 Jul;29(7):1472–1479. doi: 10.1128/jcm.29.7.1472-1479.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Macchi B., Robert-Guroff M., Gallo R. C. Transformation of different phenotypic types of human bone marrow T-lymphocytes by HTLV-1. Int J Cancer. 1984 Jan 15;33(1):13–17. doi: 10.1002/ijc.2910330104. [DOI] [PubMed] [Google Scholar]
- Meignan M., Guillon J. M., Denis M., Joly P., Rosso J., Carette M. F., Baud L., Parquin F., Plata F., Debre P. Increased lung epithelial permeability in HIV-infected patients with isolated cytotoxic T-lymphocytic alveolitis. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1241–1248. doi: 10.1164/ajrccm/141.5_Pt_1.1241. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Georgsson G., Pálsson P. A., Najjar J. A., Lutley R., Pétursson G. Experimental visna in Icelandic sheep: the prototype lentiviral infection. Rev Infect Dis. 1985 Jan-Feb;7(1):75–82. doi: 10.1093/clinids/7.1.75. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Panitch H., Palsson P. A., Petursson G., Georgsson G. Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab Invest. 1976 Nov;35(5):444–451. [PubMed] [Google Scholar]
- Olafsson K., Smith M. S., Marshburn P., Carter S. G., Haskill S. Variation of HIV infectibility of macrophages as a function of donor, stage of differentiation, and site of origin. J Acquir Immune Defic Syndr. 1991;4(2):154–164. [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- Rubinstein A., Morecki R., Silverman B., Charytan M., Krieger B. Z., Andiman W., Ziprkowski M. N., Goldman H. Pulmonary disease in children with acquired immune deficiency syndrome and AIDS-related complex. J Pediatr. 1986 Apr;108(4):498–503. doi: 10.1016/s0022-3476(86)80822-8. [DOI] [PubMed] [Google Scholar]
- Travis W. D., Fox C. H., Devaney K. O., Weiss L. M., O'Leary T. J., Ognibene F. P., Suffredini A. F., Rosen M. J., Cohen M. B., Shelhamer J. Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis. Hum Pathol. 1992 May;23(5):529–541. doi: 10.1016/0046-8177(92)90130-u. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Lacroix-Ciaudo C., Blanche S., Cumont M. C., Henin D., Gray F., Boccon-Gibod L., Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992 Jan;140(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Turner R. R., Shiurba R. A., Eng L., Warnke R. A. Human dendritic cells and macrophages. In situ immunophenotypic definition of subsets that exhibit specific morphologic and microenvironmental characteristics. Am J Pathol. 1985 Apr;119(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Yamada O., Matsumoto T., Sasoaka R., Kurimura T. Variations in growth capacity of HIV in peripheral blood mononuclear cell preparations from different individuals. AIDS. 1990 Jan;4(1):35–40. doi: 10.1097/00002030-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Zink M. C., Yager J. A., Myers J. D. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am J Pathol. 1990 Apr;136(4):843–854. [PMC free article] [PubMed] [Google Scholar]