Abstract
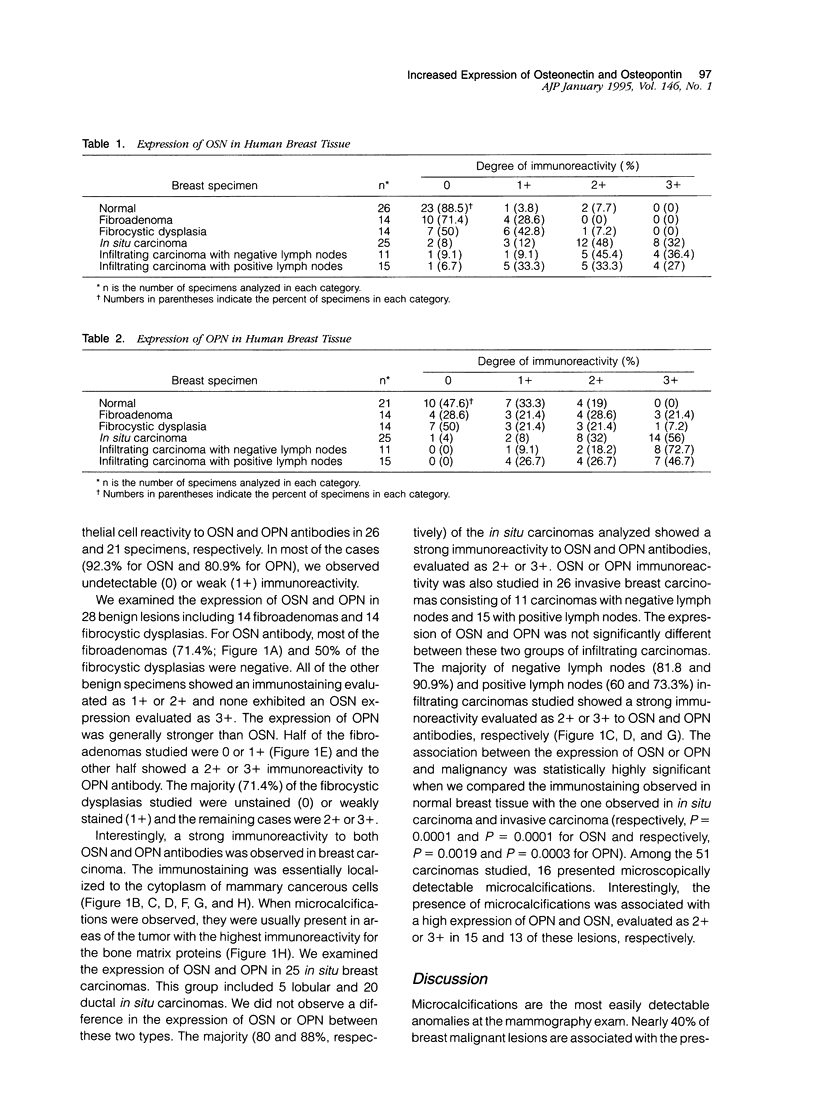
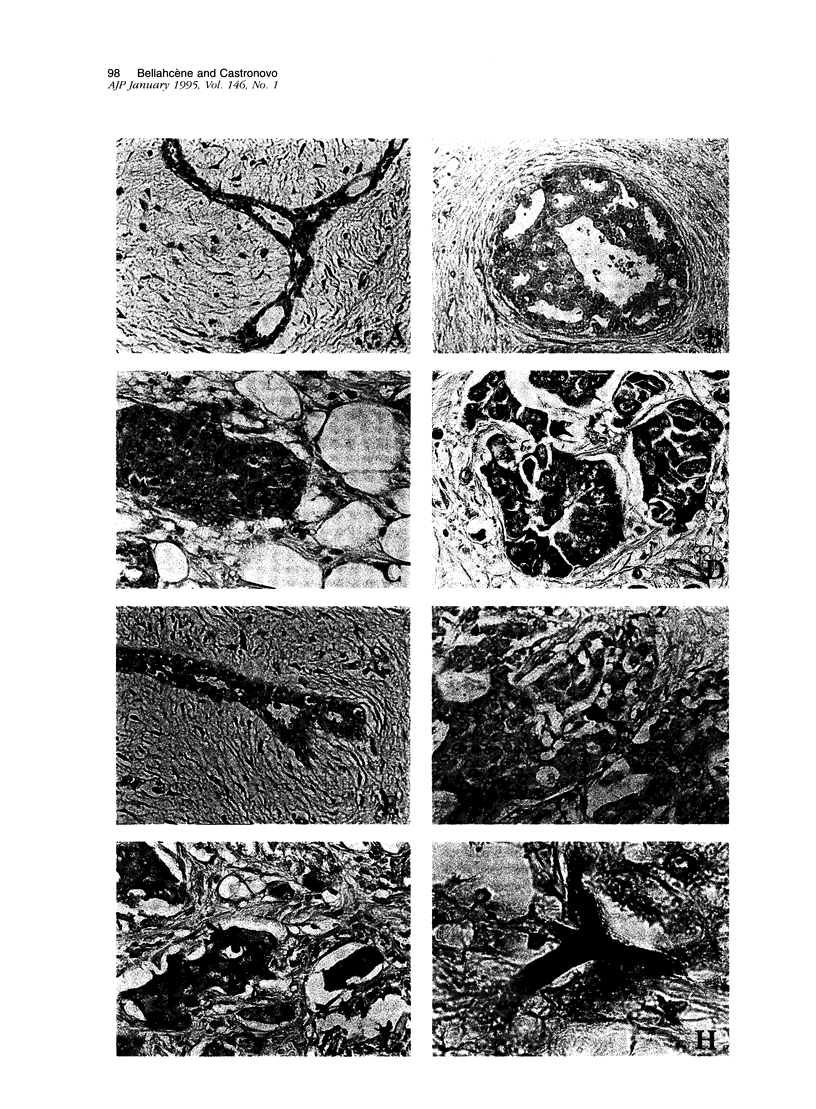
Microcalcifications are a common phenomenon associated with breast cancer and are often the only mammographic sign of a malignant breast disease. Although microcalcifications are not restricted to breast cancer and can be also associated with benign lesions, it is noteworthy that they are composed exclusively of hydroxyapatite in breast carcinoma. Hydroxyapatite is the bone-associated phosphocalcic crystal the deposition of which in bone tissue requires the coordinated expression of several molecules such as osteonectin (OSN) and osteopontin (OPN), synthesized by cells of the osteoblastic lineage. In this study, we evaluated the expression of these two bone matrix proteins, using an immunoperoxidase technique and specific antibodies, in 79 breast lesions including 28 benign and 51 cancerous specimens. We found that normal mammary tissue associated with the lesions examined expressed generally undetectable or lightly detectable (0 or 1+) amounts of OSN and OPN (92 and 81%, respectively). Benign breast lesions, including fibroadenoma and fibrocystic dysplasia, were generally weakly stained (0 or 1+) with both anti-OSN and anti-OPN antibodies (96.4 and 60.7%, respectively). Interestingly, the majority of both in situ and invasive breast carcinoma lesions showed a strong expression (2+ or 3+) for OSN or OPN (74.5 and 84.3%, respectively). High expression of these two bone matrix proteins was associated with frequent microcalcification deposition in the lesion. This study is the first extensive study of OSN and OPN expression in mammary cancers. Our data suggest that OSN and OPN could play a role in the formation of ectopic microcalcifications often associated with breast cancer. It is also tempting to speculate that the expression of these two glycoproteins by breast cancer cells play a role in the preferred bone homing of breast metastases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrend E. I., Craig A. M., Wilson S. M., Denhardt D. T., Chambers A. F. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994 Feb 1;54(3):832–837. [PubMed] [Google Scholar]
- Bellahcène A., Merville M. P., Castronovo V. Expression of bone sialoprotein, a bone matrix protein, in human breast cancer. Cancer Res. 1994 Jun 1;54(11):2823–2826. [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Denhardt G. H., Wilson S. M. ras-transformed NIH 3T3 cell lines, selected for metastatic ability in chick embryos, have increased proportions of p21-expressing cells and are metastatic in nude mice. Invasion Metastasis. 1990;10(4):225–240. [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Chen Y., Bal B. S., Gorski J. P. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992 Dec 5;267(34):24871–24878. [PubMed] [Google Scholar]
- Craig A. M., Nemir M., Mukherjee B. B., Chambers A. F., Denhardt D. T. Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):166–173. doi: 10.1016/s0006-291x(88)80028-7. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Gehron Robey P. The biochemistry of bone. Endocrinol Metab Clin North Am. 1989 Dec;18(4):858–902. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F. P., Chappel J., Alvarez J. I., Sander D., Butler W. T., Farach-Carson M. C., Mintz K. A., Robey P. G., Teitelbaum S. L., Cheresh D. A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993 May 5;268(13):9901–9907. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Asch B. B., Smith B. D., Perruzzi C. A., Dvorak H. F. A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells. Nature. 1983 Apr 21;302(5910):714–715. doi: 10.1038/302714a0. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Albrechtsen R., Fisher L. W., Young M. F., Termine J. D. Osteonectin/SPARC/BM-40 in human decidua and carcinoma, tissues characterized by de novo formation of basement membrane. Am J Pathol. 1988 Aug;132(2):345–355. [PMC free article] [PubMed] [Google Scholar]