Abstract
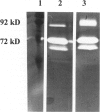
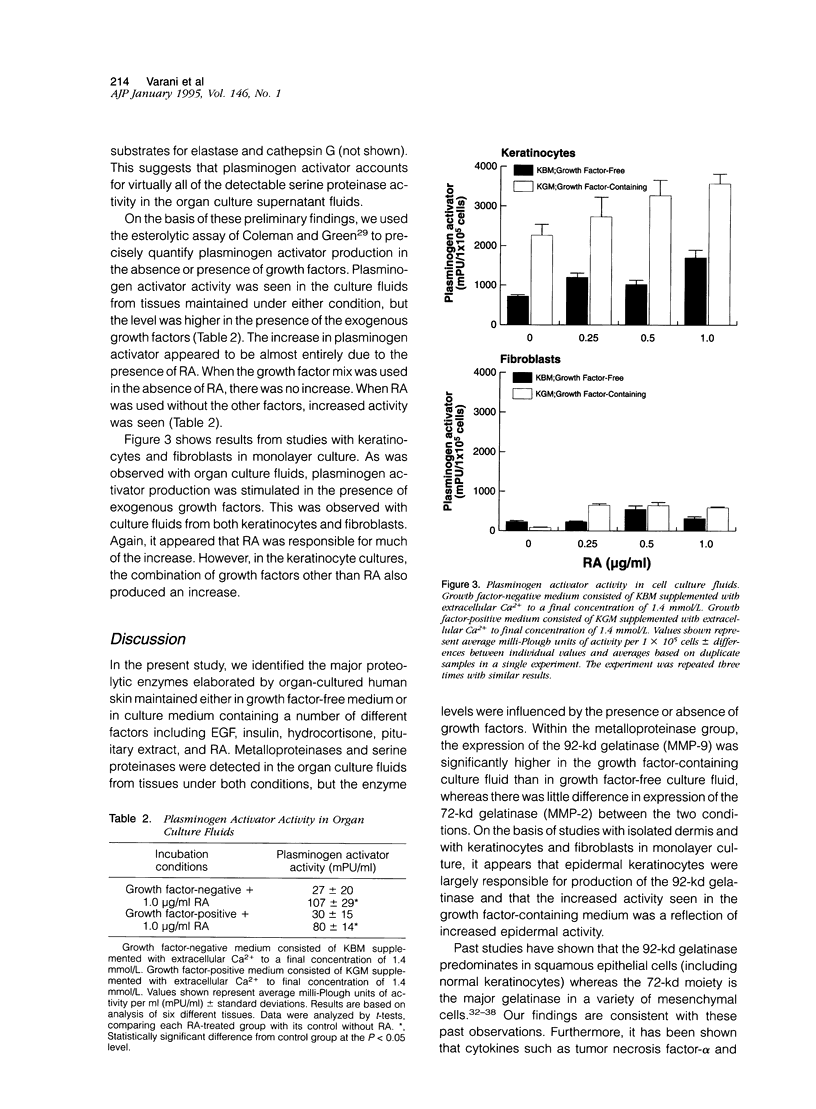
Proteinase levels were assessed in organ culture fluids from human neonatal foreskin maintained under growth factor-free conditions and in the presence of a combination of growth factors (ie, epidermal growth factor, insulin, hydrocortisone, pituitary extract, and all-trans-retinoic acid). Analysis of culture fluids by gelatin zymography revealed the presence of 92-kd and 72-kd gelatinases. There was a greater amount of 92-kd gelatinase activity in the presence of growth factors whereas the levels of 72-kd gelatinase were similar in growth factor-free and growth factor-containing media. Experiments with keratinocytes and fibroblasts in monolayer culture and with isolated dermal tissue in organ culture indicated that the epithelial component was responsible for most of the 92-kd gelatinase activity whereas fibroblasts were primarily responsible for the 72-kd gelatinase activity. Activation with aminophenyl mercuric acetate, requirement for divalent cations, inhibition with EDTA, and insensitivity to inhibition with phenylmethyl sulfonyl fluoride indicated that both gelatinases were metalloproteinases. In additional studies, culture fluids were examined for the presence of plasminogen activator activity. This was detected in culture fluids from tissues maintained under both conditions but was increased in the growth factor-containing medium. The increased amount seen in the growth factor-containing medium appeared to be due almost entirely to a single factor, ie, all-trans-retinoic acid. In monolayer culture, both keratinocytes and fibroblasts produced plasminogen activator; the level was higher in keratinocyte culture fluids than in culture fluids from fibroblasts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal C., Hembree J. R., Rorke E. A., Eckert R. L. Transforming growth factor beta 1 regulation of metalloproteinase production in cultured human cervical epithelial cells. Cancer Res. 1994 Feb 15;54(4):943–949. [PubMed] [Google Scholar]
- Albini A., Melchiori A., Santi L., Liotta L. A., Brown P. D., Stetler-Stevenson W. G. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991 Jun 5;83(11):775–779. doi: 10.1093/jnci/83.11.775. [DOI] [PubMed] [Google Scholar]
- Bailly C., Drèze S., Asselineau D., Nusgens B., Lapière C. M., Darmon M. Retinoic acid inhibits the production of collagenase by human epidermal keratinocytes. J Invest Dermatol. 1990 Jan;94(1):47–51. doi: 10.1111/1523-1747.ep12873342. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., McMillan R. M., Dayer J. M., Harris E. D., Jr Inhibition by retinoic acid of collagenase production in rheumatoid synovial cells. N Engl J Med. 1980 Aug 21;303(8):432–436. doi: 10.1056/NEJM198008213030805. [DOI] [PubMed] [Google Scholar]
- Canete-Soler R., Litzky L., Lubensky I., Muschel R. J. Localization of the 92 kd gelatinase mRNA in squamous cell and adenocarcinomas of the lung using in situ hybridization. Am J Pathol. 1994 Mar;144(3):518–527. [PMC free article] [PubMed] [Google Scholar]
- Coleman P. L., Green G. D. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann N Y Acad Sci. 1981;370:617–626. doi: 10.1111/j.1749-6632.1981.tb29768.x. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Ferriola P. C., Earp H. S., Di Augustine R., Nettesheim P. Role of TGF alpha and its receptor in proliferation of immortalized rat tracheal epithelial cells: studies with tyrphostin and TGF alpha antisera. J Cell Physiol. 1991 Apr;147(1):166–175. doi: 10.1002/jcp.1041470121. [DOI] [PubMed] [Google Scholar]
- Fligiel S. E., Lee E. C., McCoy J. P., Johnson K. J., Varani J. Protein degradation following treatment with hydrogen peroxide. Am J Pathol. 1984 Jun;115(3):418–425. [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha R., Denhardt D. T. Matrix metalloproteinases and tissue inhibitor of metalloproteinases: a review of their role in tumorigenesis and tissue invasion. Invasion Metastasis. 1989;9(6):391–405. [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Moniwa N., Gotoh J., Sugimura M., Terao T. Role of activated protein C in facilitating basement membrane invasion by tumor cells. Cancer Res. 1994 Jan 1;54(1):261–267. [PubMed] [Google Scholar]
- Kobayashi H., Shinohara H., Takeuchi K., Itoh M., Fujie M., Saitoh M., Terao T. Inhibition of the soluble and the tumor cell receptor-bound plasmin by urinary trypsin inhibitor and subsequent effects on tumor cell invasion and metastasis. Cancer Res. 1994 Feb 1;54(3):844–849. [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Mauch C., Adelmann-Grill B., Hatamochi A., Krieg T. Collagenase gene expression in fibroblasts is regulated by a three-dimensional contact with collagen. FEBS Lett. 1989 Jul 3;250(2):301–305. doi: 10.1016/0014-5793(89)80743-4. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Mulder K. M. Differential regulation of c-myc and transforming growth factor-alpha messenger RNA expression in poorly differentiated and well-differentiated colon carcinoma cells during the establishment of a quiescent state. Cancer Res. 1991 May 1;51(9):2256–2262. [PubMed] [Google Scholar]
- Mulligan M. S., Desrochers P. E., Chinnaiyan A. M., Gibbs D. F., Varani J., Johnson K. J., Weiss S. J. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11523–11527. doi: 10.1073/pnas.90.24.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. J., Murphy G., Reynolds J. J. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991 Sep 2;289(1):4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- Oikarinen A., Kylmäniemi M., Autio-Harmainen H., Autio P., Salo T. Demonstration of 72-kDa and 92-kDa forms of type IV collagenase in human skin: variable expression in various blistering diseases, induction during re-epithelialization, and decrease by topical glucocorticoids. J Invest Dermatol. 1993 Aug;101(2):205–210. doi: 10.1111/1523-1747.ep12363823. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Paganetti P. A., Caroni P., Schwab M. E. Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol. 1988 Dec;107(6 Pt 1):2281–2291. doi: 10.1083/jcb.107.6.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood S. M., Liu B. C., Weiss R. E., Hodge D. E., Droller M. J. Abrogation of the invasion of human bladder tumor cells by using protease inhibitor(s). Cancer. 1992 Mar 1;69(5):1212–1219. doi: 10.1002/cncr.2820690524. [DOI] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Rice W. G., Weiss S. J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990 Jul 13;249(4965):178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B. Plasminogen activator expression and matrix degradation. Matrix Suppl. 1992;1:20–22. [PubMed] [Google Scholar]
- Salo T., Lyons J. G., Rahemtulla F., Birkedal-Hansen H., Larjava H. Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem. 1991 Jun 25;266(18):11436–11441. [PubMed] [Google Scholar]
- Schultz R. M., Silberman S., Persky B., Bajkowski A. S., Carmichael D. F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988 Oct 1;48(19):5539–5545. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Shapiro S. D., Kobayashi D. K., Pentland A. P., Welgus H. G. Induction of macrophage metalloproteinases by extracellular matrix. Evidence for enzyme- and substrate-specific responses involving prostaglandin-dependent mechanisms. J Biol Chem. 1993 Apr 15;268(11):8170–8175. [PubMed] [Google Scholar]
- Sloane B. F., Dunn J. R., Honn K. V. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981 Jun 5;212(4499):1151–1153. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- Varani J., Burmeister B., Sitrin R. G., Shollenberger S. B., Inman D. R., Fligiel S. E., Gibbs D. F., Johnson K. Expression of serine proteinases and metalloproteinases in organ-cultured human skin. Altered levels in the presence of retinoic acid and possible relationship to retinoid-induced loss of epidermal cohesion. Am J Pathol. 1994 Sep;145(3):561–573. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Perone P., Inman D. R., Voorhees J. J. Effects of sodium lauryl sulfate on human skin in organ culture: comparison with all-trans-retinoic acid and epidermal growth factor. Dermatology. 1993;187(1):19–25. doi: 10.1159/000247191. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Schuger L., Perone P., Inman D., Griffiths C. E., Voorhees J. J. Effects of all-trans retinoic acid and Ca++ on human skin in organ culture. Am J Pathol. 1993 Jan;142(1):189–198. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Larson B. K., Perone P., Inman D. R., Fligiel S. E., Voorhees J. J. All-trans retinoic acid and extracellular Ca2+ differentially influence extracellular matrix production by human skin in organ culture. Am J Pathol. 1993 Jun;142(6):1813–1822. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Perone P., Fligiel S. E., Inman D. R., Voorhees J. J. all-trans-retinoic acid preserves viability of fibroblasts and keratinocytes in full-thickness human skin and fibroblasts in isolated dermis in organ culture. Arch Dermatol Res. 1994;286(8):443–447. doi: 10.1007/BF00371569. [DOI] [PubMed] [Google Scholar]
- Varani J., Perone P., Griffiths C. E., Inman D. R., Fligiel S. E., Voorhees J. J. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest. 1994 Nov;94(5):1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Shayevitz J., Perry D., Mitra R. S., Nickoloff B. J., Voorhees J. J. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990 Jun;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Wang M., Stearns M. E. Blocking of collagenase secretion by estramustine during in vitro tumor cell invasion. Cancer Res. 1988 Nov 15;48(22):6262–6271. [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Xie B., Bucana C. D., Fidler I. J. Density-dependent induction of 92-kd type IV collagenase activity in cultures of A431 human epidermoid carcinoma cells. Am J Pathol. 1994 May;144(5):1058–1067. [PMC free article] [PubMed] [Google Scholar]
- Yagel S., Khokha R., Denhardt D. T., Kerbel R. S., Parhar R. S., Lala P. K. Mechanisms of cellular invasiveness: a comparison of amnion invasion in vitro and metastatic behavior in vivo. J Natl Cancer Inst. 1989 May 10;81(10):768–775. doi: 10.1093/jnci/81.10.768. [DOI] [PubMed] [Google Scholar]
- de Vries T. J., Quax P. H., Denijn M., Verrijp K. N., Verheijen J. H., Verspaget H. W., Weidle U. H., Ruiter D. J., van Muijen G. N. Plasminogen activators, their inhibitors, and urokinase receptor emerge in late stages of melanocytic tumor progression. Am J Pathol. 1994 Jan;144(1):70–81. [PMC free article] [PubMed] [Google Scholar]