Abstract
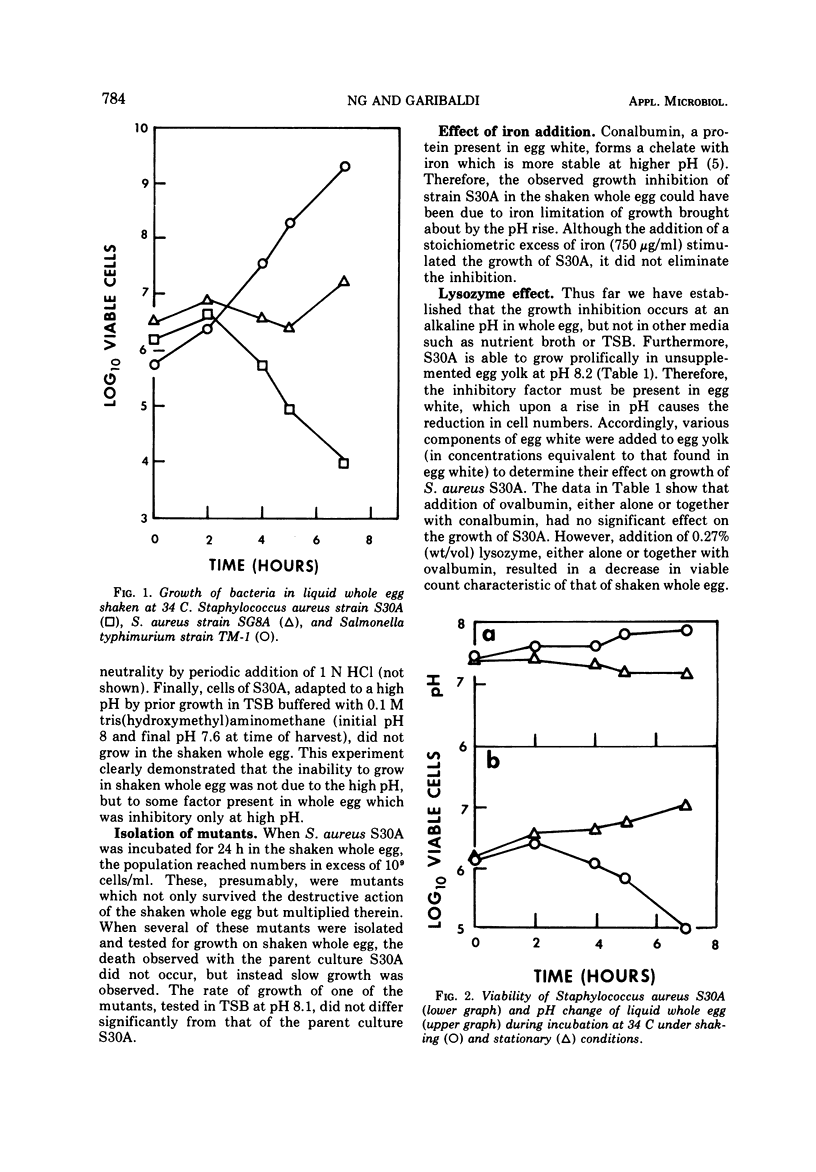
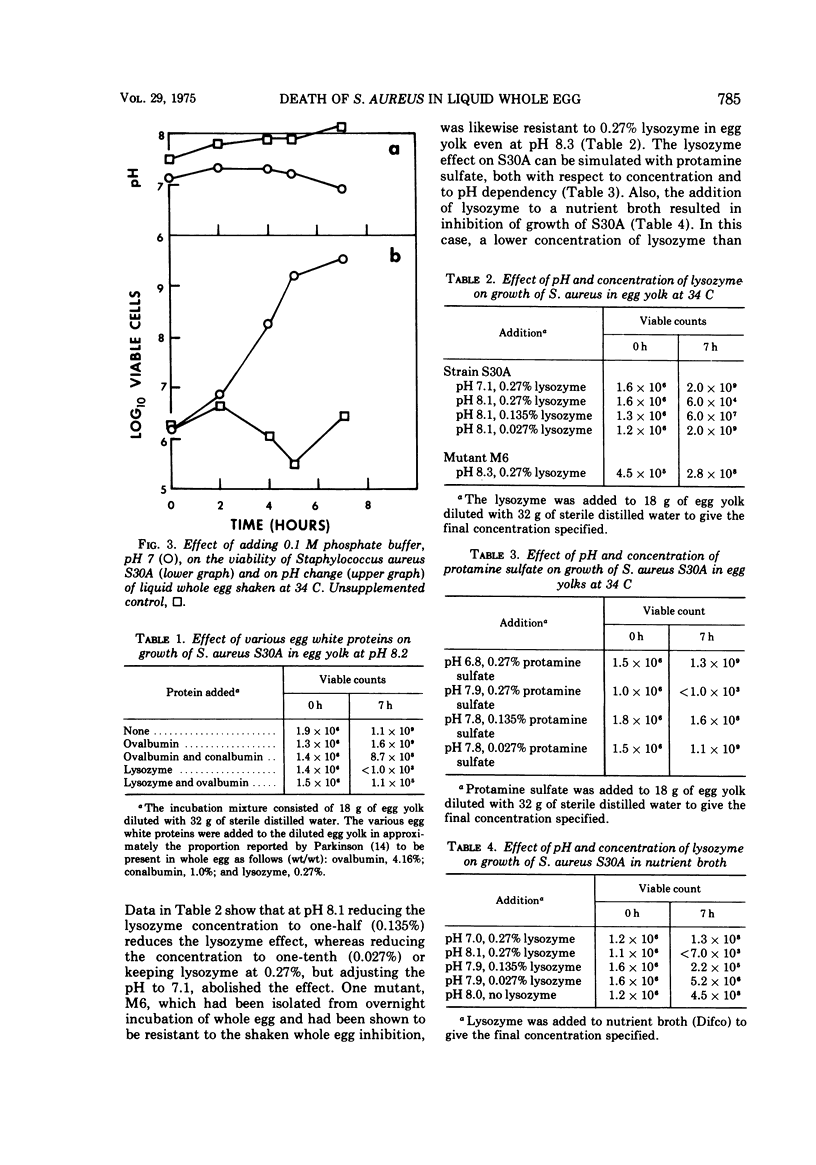
Incubating and shaking Staphylococcus aureus in liquid whole egg causes a decline in viability. During the period of agitation, the natural pH of the egg rises from about 7.2 to between 8.0 and 8.2 as a result of a loss of carbon dioxide. However, if the pH of the egg is prevented from rising, either by not shaking or by addition of a buffer, S. aureus will grow. The cause of death is traced to the presence of lysozyme of egg white. Interestingly, the action of lysozyme is not attributable to its bacterial lytic property but, instead, to the basicity of the lysozyme molecule. This conclusion is supported by the fact that the lytic property of lysozyme is known to have its optimal activity near neutrality and by the finding that protamine sulfate, a nonenzymatic basic polypeptide, also caused death of S. aureus at pH 8.0 but not at 7.0. It was postulated that the rise in pH renders the bacterial cells more negatively charged, so that in the presence of positively charged molecules like lysozyme or protamine sulfate a complex is formed, agglutinating the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELOTTI R., FOTER M. J., LEWIS K. H. Time-temperature effects on Salmonellae and Staphylococci in foods. III. Thermal death time studies. Appl Microbiol. 1961 Jul;9:308–315. doi: 10.1128/am.9.4.308-315.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board R. G. Review article: the course of microbial infection of the hen's egg. J Appl Bacteriol. 1966 Aug;29(2):319–341. doi: 10.1111/j.1365-2672.1966.tb03482.x. [DOI] [PubMed] [Google Scholar]
- FEVOLD H. L. Egg proteins. Adv Protein Chem. 1951;6:187–252. doi: 10.1016/s0065-3233(08)60504-5. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A., Cannan R. K. The hydrogen ion dissociation curve of the crystalline albumin of the hen's egg. Biochem J. 1936 Feb;30(2):227–234. doi: 10.1042/bj0300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. F., Abrams R., Dorfman A., Klein M. ANTIBACTERIAL PROPERTIES OF PROTAMINE AND HISTONE. Science. 1942 Nov 6;96(2497):428–430. doi: 10.1126/science.96.2497.428. [DOI] [PubMed] [Google Scholar]
- Parkinson T. L. The chemical composition of eggs. J Sci Food Agric. 1966 Mar;17(3):101–111. doi: 10.1002/jsfa.2740170301. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The properties of lysozyme and its action on microorganisms. Bacteriol Rev. 1957 Jun;21(2):82–100. doi: 10.1128/br.21.2.82-100.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. The inhibition of lysozyme by acidic polymers from pathogenic bacteria. J Bacteriol. 1955 Jul;70(1):110–112. doi: 10.1128/jb.70.1.110-112.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. T., White J. C., Longrée K. Thermal resistance of salmonellae and staphylococci in foods. Appl Microbiol. 1966 Sep;14(5):815–820. doi: 10.1128/am.14.5.815-820.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



