Abstract
The saliva of bloodsucking arthropods contains a large array of pharmacologically active compounds that assist hematophagy. Arthropod saliva is also responsible for causing uncomfortable allergic responses in its vertebrate hosts. In this article, we investigate whether the sand fly Phlebotomus papatasi, known to produce a strong delayed-type hypersensitivity (DTH) in humans, could benefit from, and possibly adaptively induce, this response in their vertebrate hosts. In this study, we show that flies fed on humans to completion nearly twice as fast in DTH sites as compared with normal skin sites. DTH sites had significantly larger blood flow as measured by the laser Doppler method. Sand flies feeding at sites in mouse ears that had a DTH response also fed faster than at normal sites. We conclude that in the case of P. papatasi, and possibly other arthropods such as fleas and bed bugs, the strong saliva-induced DTH response may reflect an adaptation of the fly to manipulate host immunity for the insect's own advantage.
In their quest for blood, hematophagous arthropods have evolved a sophisticated array of salivary pharmacological components that counteract hemostasis and inflammatory reactions of the vertebrate hosts. Indeed, several different types of salivary anticlotting, antiplatelet, vasodilatory, and immunosuppressive activities have been described in the past 15 years, many of which have been characterized molecularly (1, 2). This more recent literature contrasts with previous literature, emphasizing the role of saliva in inducing host allergic response (3–5).
The natural history of hypersensitivity to mosquito bites was first proposed by Mellanby (6). Naive hosts develop no reaction or a delayed-type hypersensitivity (DTH) reaction when first exposed to bites, evolving to immediate-type hypersensitivity (IT) and desensitization as exposure is continued, such as DTH → DTH + IT → IT → Desensitization. This set of responses is also similar for other blood-feeding arthropods, such as fleas (7) and bed bugs (8). Such responses have been interpreted as noxious to the blood feeder. Increased host grooming and defensive behavior triggered by IT may disrupt blood feeding (4). Additionally, effective host immune responses can be mounted against ticks, which take several days to complete their blood meals (9, 10).
Considering that bloodsucking arthropods have an extremely sophisticated pharmacologic armamentarium—and that the host immune responses against salivary antigens are potentially disruptive to blood feeding—one can question why such insects, in their pharmacologic wisdom, “allow” this response to occur. There are several possibilities, none mutually exclusive: (i) Host response may not matter at all, as in the long run most will be desensitized; only a minority of hosts will display allergic responses at any one time. This scenario may be true for those arthropods occurring and feeding all year long, without marked seasonality, on a long-lived vertebrate. (ii) Host response may be disruptive to blood feeding, and salivary immunosuppressors may be produced, as in the case of ticks (11) and the New World sand fly Lutzomyia longipalpis, which have potent salivary immunosuppressive activities (12–14). (iii) Allergic host responses may be of interest to the insect, as it may increase blood flow at the site of feeding. In this case, salivary components may be specifically produced to exacerbate such host skin responses. DTH responses, in particular, may be exploited by those insects feeding repeatedly on the same host with an interval of 24–72 h, such as some sand flies and bed bugs.
The Old World sand fly Phlebotomus papatasi produces a remarkable skin long-lasting response. Indeed, a clinical entity known as “harara” was described more than 60 years ago afflicting Palestine immigrants (15). P. papatasi lives in the burrows of rodents. After a blood meal, it develops eggs and returns to feed within 48–72 h (16). Sand flies feeding on rodents have limited access to a feeding site, usually the ears, tail, or feet, portions with small surface area. This type of fly could benefit from the DTH response of the hosts. P. papatasi exist also as peridomestic populations, living within and around houses where humans are the main blood source (S.K., unpublished work). Accordingly, we investigated the effect of a host DTH response on the sand fly's feeding behavior in humans and began investigation on the molecules involved in the initiation of this DTH response using a mouse model.
Materials and Methods
Human Subjects.
We have used six adult human volunteers for this study, three with a history of DTH following bites of P. papatasi and three without a history of exposure to this fly. Protocols were approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases.
Sand Flies and Preparation of Salivary Gland Homogenates.
P. papatasi, Israeli strain, were reared at the Walter Reed Army Medical Research Institute using as larval food a mixture of fermented rabbit feces and rabbit food (17). Adults were offered a cotton swab containing 20% sucrose and were used for behavioral studies or dissection of salivary glands at 2–7 days following emergence. Salivary glands were usually stored in groups of 20 pairs in 20 μl of NaCl (150 mM) Hepes buffer (10 mM, pH 7.4). Salivary glands were disrupted by ultrasonication within 1.5-ml conical tubes. Individual tubes held by forceps were submersed in water and the tip of a Branson sonifier 450 was set immediately above the glands. Power was adjusted to immediately below the cavitation level (observed by the apparent boiling of the water). Twenty intermittent pulses of 50% duration were applied to the tube. Tubes were centrifuged at 10,000 × g for 2 min, and the resultant supernatant was used for the studies.
DTH Studies in Mice.
Female BALB/c or C57BL/6N (B6) mice, 8–12 weeks old, were purchased from the National Cancer Institute (Frederick, MD). All mice were kept in the National Institute of Allergy and Infectious Diseases animal care facility under pathogen-free conditions.
To sensitize mice with salivary gland homogenates, 0.2 pair of salivary gland sonicate (SGS) (≈200 ng protein) were inoculated intradermally into the ear dermis of C57BL/6 mice using a 27½-gauge needle in a volume of 10 μl of PBS (10 mM sodium phosphate, pH 7.0, and 150 mM NaCl) in one ear, followed by a second injection with the same amount of material 2 weeks later in the same ear. Challenge was done in the opposite ear 2 weeks after the last injection. Alternatively, mice were sensitized directly by sand fly bite. In this case, for each sensitization, emergent females, left without sugar or water, were used the following day. Ten healthy flies were placed in plastic vials, the upper surfaces of which were covered with a fine netting. Mice were anesthetized i.p. with 200 μl of 20 mg/ml ketamine HCl (Phoenix Pharmaceuticals, Mountain View, CA). A single ear from each of the anesthetized mice was pressed closely to the meshed surface of the vials, secured by clamps designed for this purpose, and flies were allowed to feed in the dark for a period of 30 min. A minimum of five fully blood-fed flies per ear were required for each sensitization.
Analysis of Sand Fly Feeding Behavior.
Observation of sand fly blood-feeding behavior on humans was done with the help of a ×5 magnification goggle. Flies were transferred from the rearing container to the observation chamber using aspirators. The observation chamber was made by cutting a 13-mm clear plastic tube at 1 cm from the open end and closing one of the ends with plastic using a soldering iron. A side opening was made to introduce the flies, which was closed by a small cotton swab. The chamber was held on the anterior face of the forearm by an elastic band. Typically, flies took from a few seconds to 2 min to start biting. The time from initial bite to the moment of first visualization of blood in the gut of the fly was recorded as probing time. The time from first visualization of blood in the gut to withdrawal of the mouth parts was recorded as feeding time. Twelve flies (and in one case eight flies) were individually applied to the anterior forearm of the volunteer and feeding parameters were measured as described above. The site of the bite was marked with indelible ink, and 22–26 h later, the experiment was repeated with the same number of flies, but the location of the feeding chamber was placed to just exclude the site of feeding of the previous day.
To measure the feeding success of sand flies on mice previously sensitized (or naive controls) to salivary homogenate or to sand fly bite, we anesthetized mice with a mixture of ketamine HCl and placed the mice on a slide warmer kept at 37°C. For each feeding time point, 2- to 4-day-old flies were starved for 24 h and 10–12 females were placed in meshed vials ready for use. Groups of 10–12 sand flies were introduced into small cages and these were applied to the surface of an ear that was injected 24 h earlier with 0.2 pairs of homogenized salivary glands. Flies were allowed to feed for 10 min, removed, and scored as either fully fed, partially fed, or unfed. The flies used in these experiments were not blood fed previously and thus had no eggs in their abdomen. This enabled easy assessment of the size of their blood meal by visual inspection. A fully fed fly was one that had its entire abdomen full of blood, causing it to distend and acquire a bulbous shape. A partially fed fly was one that had commenced feeding, with blood clearly visible in its abdomen, but did not complete its meal. This is clearly distinguishable from a fully fed fly by the partial filling of the abdomen, the rest of which remained without color and without distension. We did not attempt to measure feeding parameters of individual flies, as done with the human volunteers because the prolonged duration of the experiment would have required a too-long period of anesthesia of mice.
Skin Blood Flow Measurements.
The laser Doppler method was carried out with a Perimed PF 5010 instrument (Perimed, North Royalton, OH) using an angular probe for human forearm skin measurement or an integrative probe (containing seven individual optical fibers in one probe) to measure blood flow in the mouse ear. For human measurements, 6–8 measurements were taken 22–26 h after sand fly exposure to a forearm skin site, at a 5 mm distance from the bitten site; control sites were measured at least 4 cm distant from the closest bite site. For measurements made on mice, three different measurements were taken at three different sites of each ear for each mouse to decrease the variance associated with the spatially irregular blood flow in this organ. Each probe was calibrated with a standard suspension of latex particles as suggested by the manufacturer.
Analysis of Inflammatory Cells in the Ear Dermis.
The cells in the inflammatory ear dermis were recovered as described (18). In brief, 18 h after intradermal inoculation, the ears were collected and rinsed in 70% ethanol with vigorous shaking and left to dry. Using a pair of fine forceps, the ventral and dorsal dermal sheets were separated and immediately processed. The two leaflets were transferred, dermal side down on culture medium. The cell populations spontaneously emigrating out of the dermal layers were recovered and filtered through a 70-μm nylon cell strainer (Becton Dickinson).
Immunolabeling for Flow Cytometry Analysis.
The dermal cells were incubated with 10% normal mouse serum in PBS containing 0.1% BSA/0.01% NaN3 before being incubated with anti-Fc receptor antibody (2.4G2; PharMingen). The double staining was done by using directly conjugated antibodies incubated simultaneously. The dermal inflammatory cells were identified by characteristic size (forward light scatter) and granulosity (side light scatter) combined with two-color analysis, as described (18). Briefly, the dendritic leukocytes were identified as large cells, MHC class II (25-9-17; PharMingen) bright and F4/80 (A3-1; Caltag, Burlingame, CA) positive. The mononuclear phagocytes were identified by F4/80 positive and MHC class II low or negative. The neutrophils were identified as small cells, Ly-6G bright (RB6-8C5; PharMingen) and negative for F4/80 (or MHC class II); the eosinophils by their granulosity associated with F4/80 lightly positive and MHC class II negative. The CD4 lymphocytes were identified by their small size and by CD4 (RM4-5; PharMingen), and CD3 (145-2C11; PharMingen) expression. The isotype controls used were rat IgG2b (A95-1; PharMingen) and rat IgG2a (R35-95; PharMingen). After staining, the cells were fixed with 4% paraformaldehyde and washed. For each sample, 10,000 cells were analyzed. Each sample was cytospun in parallel and stained with Diff-Quik solution (Harleco, Gibbstown, NJ); the percentages of neutrophils, eosinophils, and mononuclear phagocytes estimated by microscopic examination of stained cells were similar to those obtained by flow cytometry. The data were collected and analyzed by using cellquest software and a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems).
CD4 Cell Depletion.
For CD4 depletion, mice were inoculated at the time of challenge i.p. with 1 mg of anti-CD4 (GK1.5) or the appropriate isotype control (R35-38). Cells for monoclonal antibody production were obtained from the American Type Culture Collection.
Reverse-Phase HPLC of Salivary Gland Homogenate.
Reverse-phase HPLC was carried out using a PRP-3 (2 mm × 15 cm) from Hamilton and perfused with a gradient from 10 to 60% acetonitrile in water containing 8 mM HCl. Acetonitrile was of HPLC grade. A 0.8 M solution of HCl was passed through a 1-ml SepPak C18 cartridge (Waters) after activation with acetonitrile and water, to remove impurities, and used in the HPLC solutions immediately after purification. A ThermoSeparation Products (Rivera Beach, FL) CM 4100 pump and SM4100 detector were used. Salivary gland homogenate prepared from 100 pairs was applied to the column and fractions were collected at 1-min intervals and were dried on a SpeedVac (Savant) in the presence of 10 μg of BSA. After reconstitution in 100 μl of 150 mM NaCl and 10 mM Hepes, pH 7.4 (Hepes saline), fractions were pooled in groups of two and 10 μl was injected in the mouse ear for assays. After identification of active pool(s), individual aliquots were similarly tested, after 10-fold dilution in Hepes saline.
Statistical Analyses.
sigmastat (Jandel Software, San Raphael, CA) was used to perform statistical tests. Whenever variances were homogeneous, t tests were used; otherwise, Kruskal–Wallis tests or χ2 tests were used, as indicated.
Results
Influence of DTH on Blood Flow and Sand Fly Feeding Behavior in Humans.
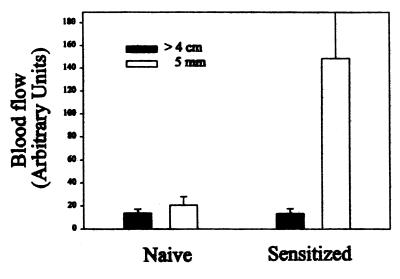
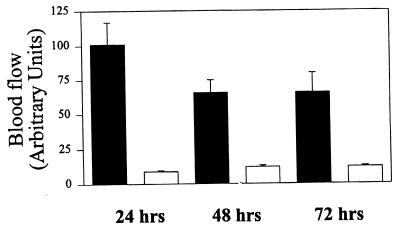
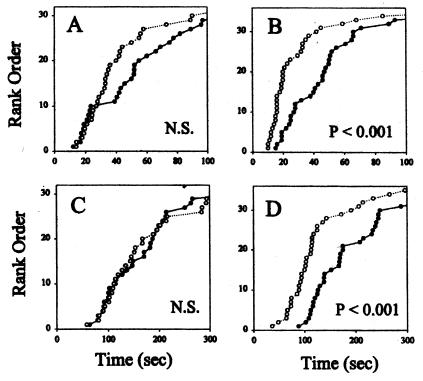
When P. papatasi fed on previously sensitized human subjects, a typical DTH lesion developed after the bite. The DTH reaction in sensitized individuals started 6–8 h after the bite, characterized by itching, redness of the site, and induration. As expected, on individuals without a previous history of exposure to the sand fly, the feeding lesions were almost unnoticed 24 h after the bite. In the sensitized individuals, blood flow near the feeding sites increased 10-fold 24 h after the bite, as measured by the laser Doppler technique (Fig. 1). No increase in blood flow was found near the feeding sites in the naive individuals. Although there was a decrease in blood flow in the sensitized individuals from 24 to 48 h postbite, there was still significantly larger blood flow at 72 h compared with control sites on the contralateral arm (Fig. 2). Sand fly probing time as well as feeding time were significantly decreased 24 h after sand fly exposure in sensitized individuals; however, these differences were not significant in nonsensitized individuals (Fig. 3). Taken together, a 40.1% ± 4.8 (mean ± SE, n = 3) reduction in total time to accomplish feeding (median probing time + median feeding time) was observed only in sensitized individuals. Corresponding values for nonsensitized individuals were 3.47 ± 10.9 percentage change. The differences in average change of total blood-feeding time among sensitized and nonsensitized individuals were significant at P = 0.018 when compared by a t test.
Figure 1.
Blood flow (measured as arbitrary units) at skin sites >4 cm distant or at 5 mm from sites fed 22–26 h earlier by P. papatasi sand flies in sensitized individuals (preexposed) or nonsensitized (naive) individuals. The bars are the average ± SE of three individuals from whom six to eight measurements were taken.
Figure 2.
Blood flow (measured as arbitrary units) at skin sites of one individual displaying DTH to P. papatasi measured at different times after insect bite on an anterior forearm. Control sites were measured in the contralateral arm. The bars are the average ± SE of 6–8 skin site measurements. Filled bars represent DTH sites and open bars represent control sites.
Figure 3.
Probing (A and B) and feeding (C and D) times of P. papatasi feeding on the anterior forearm of humans not previously exposed to sand fly (A and C) or to forearms of humans displaying a DTH reaction to sand fly bite (B and D). Measurements were made in normal skin sites (●) or near (5 mm) skin sites where sand flies fed 22–26 h before (○). The graphs represent the rank order of the cumulative results using three volunteers displaying DTH response to the sand fly bite and three nonsensitized volunteers. A nonparametric Kruskal–Wallis test gave the P values shown in the graphs. NS, nonsignificant results.
Mouse Models of “Harara.”
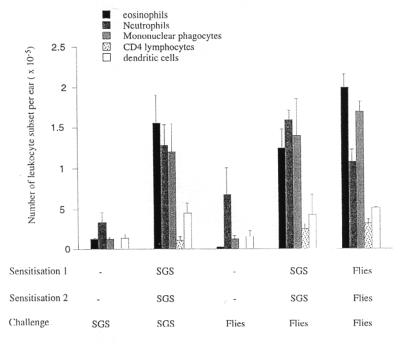
To further investigate the nature of the DTH induced by P. papatasi in their vertebrate hosts, we developed a mouse model of harara. Mice were presensitized twice by intradermal injection of 0.2 SGS (2-week intervals) on the right ear or exposed to the bite of sand flies. Two weeks later, the mice were challenged on the dermis of the left ear with the same dose of SGS (Fig. 4). The tissue response is characterized by a swelling reaching a 3-fold increase in the ear thickness with a maximum around 24 h postchallenge (data not shown). The cellular response in sensitized mice, induced by salivary gland homogenate on mice presensitized by SGS, was characterized by accumulation of a number of leukocyte subsets. At 18 h after the challenge, the infiltrate was composed of monocytes/macrophages (a 10-fold increase vs. the control ear), eosinophils (13-fold increase), neutrophils (3.5-fold increase), dendritic cells (3.4-fold increase), and CD4 lymphocytes (82-fold increase) (Fig. 4). The number of cells peaked between 18 and 48 h after the challenge but was still significantly higher than the control for a period of 5 days (data not shown). To demonstrate that this response was also true following a sand fly bite, the mice were also presensitized either by SGS or by bite and then exposed to sand flies. The nature and the number of cells sedimenting from the ears of mice sensitized by bite and challenged by bite or SGS were comparable (Fig. 4), demonstrating that the SGS is an appropriate source of antigen to mimic the natural response due to the sand fly bite.
Figure 4.
Leukocytes present in the dermal compartment 18 h after intradermal inoculation of 0.2 pair of SGS or sand fly bite in the ear of C57BL/6 mice, naive, or preexposed to SGS or sand fly bite. The cells obtained from six ears per group were pooled, and the different populations were identified by staining and flow cytometry as described in Materials and Methods. The data shown are the means ± 1 SD of three separate experiments.
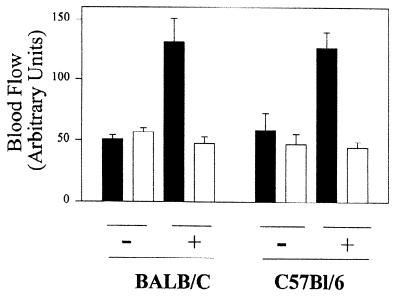
When sensitized mice were depleted in CD4+ cells using an anti-CD4 antibody at the time of the challenge, the cellular infiltrate due to the challenge with SGS was abolished, confirming the classical T cell dependence of the DTH response. The number of eosinophils and macrophages mobilized in the dermis of challenged C57BL/6 mice was reduced by 86% and 75%, respectively, compared with the control sensitized mice (Fig. 5). This indicated that CD4 T lymphocytes are the primary effector of this response. Similar to sensitized humans, sensitized but not naive mice developed increased ear blood flow 24 h postinjection of salivary homogenate (Fig. 6). This experiment also indicated that persistent (>24 h postinjection) increase in ear blood flow was found only after sensitization, thus excluding the participation of a long-lasting salivary vasodilator. We conclude—based on the kinetics and the nature of the cellular accumulation, the tissue swelling, the dependence on CD4 T lymphocytes, and the important vasodilation—that salivary gland homogenates from P. papatasi induce a strong DTH response in mice.
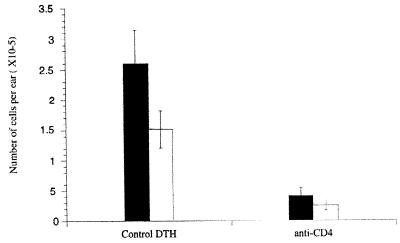
Figure 5.
Effects of anti-CD4 treatment on neutralizing the influx of eosinophil (■) and mononuclear phagocyte (□) infiltrates on ears of C57BL/6 mice previously sensitized with P. papatasi, salivary gland sonication, and challenged by injection 18 h before the cells were harvested. Mice were treated, or not, with anti-CD4 at the time of the challenge. The data shown are representative of two separate experiments.
Figure 6.
Ear blood flow in previously sensitized (+) or control (−) (not previously sensitized) mice injected 24 h before with 0.2 pairs of P. papatasi salivary gland homogenate (≈200 ng protein). In each group, the contralateral ear (open bar) was injected with saline alone. Filled bars indicate ears injected with salivary homogenate.
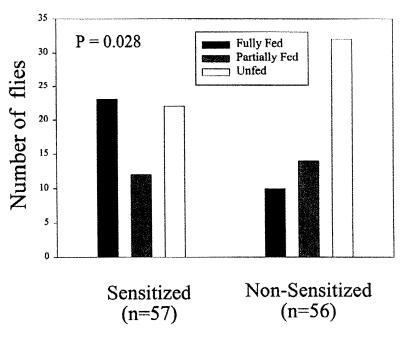
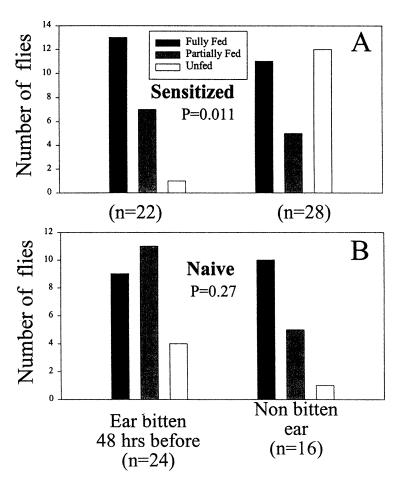
To investigate whether the DTH response in mice would increase the blood-feeding success of sand flies, we exposed sand flies to ears of previously sensitized mice or nonsensitized control mice that were challenged with SGS 18 h before on one ear. Accordingly, flies were exposed for 10 min to four anesthetized mice from each group (12 flies per ear), and their feeding success was measured as empty, partially fed, or fully fed. Combined results are presented in Fig. 7. Results indicate that flies feeding on ears of presensitized mice that were challenged 18 h before with salivary homogenate, fed significantly better (P = 0.028, χ2 test) in the 10-min period when compared with flies fed on nonsensitized ears, which were also injected 18 h previously with SGS. In another set of experiments, we investigated the feeding success of flies feeding on mice presensitized to sand fly bites, instead of SGS injection, and exposed 48 h before on the left ear to 10 sand flies. The right ear served as a non-DTH control ear in the same sensitized mouse. Results indicate that flies feeding on ears having a DTH response fed better (P = 0.011, χ2 test) than those feeding in ears that had not been exposed 48 h before to sand fly bites (Fig. 8). Flies feeding on nonsensitized mice did not statistically differ in their feeding success whether they fed on an ear bitten or not 48 h before (P = 0.27).
Figure 7.
Feeding success of P. papatasi sand flies feeding for 10 min on the ears of mice injected 18 h before with 0.2 pairs of homogenized salivary glands. Four mice were used per group (previously sensitized and naive); the number of flies are shown as n in each experimental group. Results are significantly different at P = 0.028 by a χ2 test.
Figure 8.
Feeding success of P. papatasi sand flies feeding for 10 min on the ears of mice previously sensitized by sand fly bites at 2 and 4 weeks before the experiment (A) or on the ears of naive mice (B). Three mice were used per group; the number of flies are shown as n in each experimental group. Forty-eight hours before the experiment, the left ears of both naive and sensitized mice were exposed to 10 sand flies. Results are significantly different at P = 0.011 by a χ2 test when flies fed on ears bitten 48 h before, and not significant (P = 0.27) when the flies fed on ears that were not bitten 48 h before the experiment.
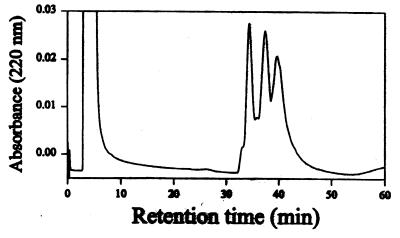
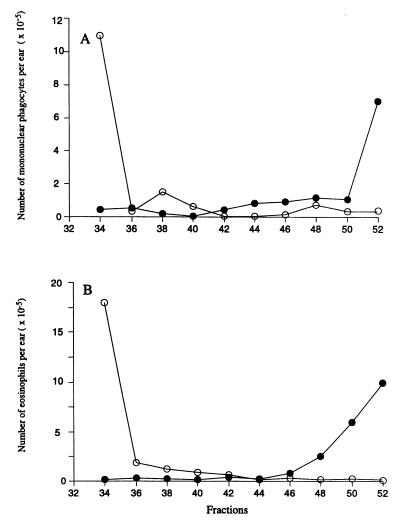
To investigate whether the P. papatasi-induced DTH response in mice was the result of multiple antigens or whether there might be one or a small number of salivary components inducing such reaction, we submitted 100 pairs of P. papatasi salivary glands to reverse-phase HPLC (Fig. 9) and assayed the fractions for their ability to induce cellular recruitment to the ears of mice previously sensitized with whole salivary homogenate. The flow cytometry analysis of the sedimenting cells, 18 h after intradermal inoculation of the different fractions, showed that BALB/c mice reacted to a different fraction of the column compared with C57BL/6 mice (Fig. 10). The first fraction injected, combining tubes with retention times ranging from 33 to 35 min, induced a strong mobilization of eosinophils (1.8 × 106/ear) (Fig. 10B), mononuclear phagocytes (1.15 × 106/ear) (Fig. 10A), and neutrophils (data not shown) in the BALB/c mice. In C57BL/6 mice, fractions 50–52 induced the mobilization of eosinophils (7 × 105) and macrophages (1.1 × 106), whereas fractions 33–35 had no effect. We conclude that only a small number of salivary molecules are responsible for the induction of the DTH response, and the response to these antigens is restricted to the immunogenetic background of the mouse.
Figure 9.
Reverse-phase HPLC of 100 pairs of P. papatasi salivary gland homogenates.
Figure 10.
Total number of monocytes/macrophages (A) and eosinophils (B) harvested from the ears of BALB/c (○) and C57BL/6 (●) mice, presensitized in the contralateral ear with P. papatasi salivary gland homogenates (with two injections of the homogenate at 2 and 4 weeks before the experiment), and challenged 18 h before dissection of the ears with indicated fractions from reverse-phase HPLC of P. papatasi salivary gland homogenate. Data represent the average number of cells in the challenged ear only, two ears per fraction.
Discussion
In this report, we demonstrated that P. papatasi induces a strong DTH response in both humans and rodents which facilitates its blood feeding. This is the first report, so far as we are aware, that a hematophagous arthropod can generate an immune response in its feeding source, otherwise thought to be disruptive to feeding, to promote its own survival. In both humans and mice, the DTH response was associated with increased blood flow, as measured by the laser Doppler method. Presumably, as a consequence of this increased blood flow, flies required significantly less time to probe and feed at DTH sites when compared with normal skin. It is also possible that the swelling associated with the accumulation of lymph in the tissue facilitates the penetration of the proboscis in the skin. In any case, flies took nearly half the time to probe and feed at human DTH sites when compared with normal skin sites.
It remains to be investigated whether these laboratory observations represent an evolutionary selected, adaptive response. Considering that P. papatasi mainly feeds on small rodent hosts with limited surface area of skin available to sand fly feeding, such as ears, tail, or feet (the majority of the skin being covered by thick fur), the probability that sand flies would come to feed on a previously fed site is high. Thus, increased feeding success on such sites due to the fly-induced DTH response is likely to be observed in nature. We have not, however, evaluated the effects of the blood meal taken in the different sites for oviposition and hatching success. A decrease of nearly 50% in total host contact, as was the case with sensitized human volunteers, may be of substantial value in increasing fitness, however, because predation during feeding is a major survival challenge for blood-feeding arthropods (19). Ultimately, it is the positive balance on costs and benefits that will select a particular salivary gland component useful for the fly in acquiring blood.
The fact that the human DTH reaction following P. papatasi sand fly bites (clinically known as harare) can be reproduced in mice provides us with a useful model to study the inflammatory response in detail and to identify the antigenic fractions capable of eliciting the DTH. Mice develop a strong DTH response after a double exposure to the equivalent of 0.2 pairs of salivary glands inoculated intradermally in the ear. The reaction is CD4+ cell dependent and is characterized by recruitment of primarily mononuclear phagocytes cells, eosinophils, and CD4 lymphocytes, suggesting that sand fly saliva elicits a relatively mixed TH1 and TH2 response. The same cellular infiltrate is mobilized in the skin after the bite of sand flies, suggesting that the SGS used to sensitize the mice is an appropriate source of antigen to mimic natural exposure to sand fly saliva. The fact that a strong DTH response to sand fly saliva can be elicited in mice also supports the possibility that the adaptive nature of the response might apply to the most common rodent host of P. papatasi in nature, namely, the sand rat Psammomys obesus.
P. papatasi is also the vector of Leishmania major, causative agent of a cutaneous form of leishmaniasis. It was previously shown that the saliva of P. papatasi might dramatically increase Leishmania infectivity (13, 18, 20). One of the main hypotheses underlying these results is that the saliva of sand flies is highly immunosuppressive (13, 21). However, the present results and those recently reported (ref. 18 and S.K., unpublished work) suggest that salivary components, whether contained in SGS and presented via needle into the skin or egested by sand flies during feeding, are highly immunogenic. Only a small subset of the molecules present in the SGS appeared to be immunogenic, in so far as only a few of the HPLC fractions were able to elicit the response in sensitized mice. However, since the response was so clearly restricted by the immunogenetic background of the host, it is likely that a larger number of salivary antigens are capable of eliciting the adaptive response in outbred host populations.
One unexpected consequence of the host DTH response that pertains not to feeding success and fly fitness per se, but to their role as vectors of leishmaniasis, has recently been revealed in studies involving the transmission of L. major by P. papatasi bite. Mice that have been presensitized to the bites of uninfected sand flies are more resistant to dermal leishmaniasis transmitted by the bite of infected flies, and this resistance appears to be nonspecifically promoted by the host DTH response to salivary antigens that is elicited within the parasite inoculation site (S.K., unpublished work). Thus, the identification and cloning of immunogenic salivary components might on the one hand be useful in desensitizing individuals with especially severe forms of harare, whereas in another context the antigens might be included as components of an antileishmanial vaccine.
In conclusion, we provide evidence in this article that the presence of immunogenic molecules in sand fly saliva capable of inducing a DTH reaction in their feeding sources may be of adaptive value for sand flies, and by extension, for other bloodsucking arthropods (e.g., fleas and bed bugs) that feed repeatedly on the same host. Defining the nature of these inflammatory reactions and of the antigens eliciting them will further our understanding of a novel feeding strategy that impacts not only the fitness of hematophagous arthropods, but also their role as disease-carrying vectors.
Abbreviations
- DTH
delayed-type hypersensitivity
- SGS
salivary gland sonicate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ribeiro J M C. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro J M C. Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- 3.Baron R W, Weintraub J. Parasitol Today. 1987;3:77–82. doi: 10.1016/0169-4758(87)90163-3. [DOI] [PubMed] [Google Scholar]
- 4.Stebbings J H., Jr Perspect Biol Med. 1974;17:233–239. doi: 10.1353/pbm.1974.0027. [DOI] [PubMed] [Google Scholar]
- 5.Wikel S K. Annu Rev Entomol. 1982;27:21–48. doi: 10.1146/annurev.en.27.010182.000321. [DOI] [PubMed] [Google Scholar]
- 6.Mellanby K. Nature (London) 1946;158:554–555. doi: 10.1038/158554c0. [DOI] [PubMed] [Google Scholar]
- 7.Hudson B W, Feingold B F, Kartman L. Exp Parasitol. 1960;9:18–24. doi: 10.1016/0014-4894(60)90005-9. [DOI] [PubMed] [Google Scholar]
- 8.Sansom J E, Reynolds N J, Peachey R D. Arch Dermatol. 1992;128:272–273. doi: 10.1001/archderm.1992.01680120148027. [DOI] [PubMed] [Google Scholar]
- 9.Wikel S K. Annu Rev Entomol. 1996;41:1–22. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- 10.Trager W. J Parasitol. 1939;25:57–81. [Google Scholar]
- 11.Wikel S, Ramachandra R N, Bergman D K. Int J Parasitol. 1994;24:59–66. doi: 10.1016/0020-7519(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 12.Theodos C M, Titus R G. Parasitol Immunol. 1993;15:481–487. doi: 10.1111/j.1365-3024.1993.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 13.Hal L R, Titus R G. J Immunol. 1995;155:3501–3506. [PubMed] [Google Scholar]
- 14.Soares M B, Titus R G, Shoemaker J R, Bozza M. J Immunol. 1998;160:1811–1816. [PubMed] [Google Scholar]
- 15.Theodor O. Trans R Soc Trop Med Hyg. 1935;29:273–284. [Google Scholar]
- 16.Killick-Kendrick R. In: Biology of the Kinetoplastida. Lumsden W, Evans D, editors. Academic Press; 1979. pp. 395–460. [Google Scholar]
- 17.Modi G B, Tesh R B. J Med Entomol. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks D L. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillett J D. Proc R Soc Ser B. 1967;167:316–329. doi: 10.1098/rspb.1967.0029. [DOI] [PubMed] [Google Scholar]
- 20.Theodos C M, Ribeiro J M C, Titus R G. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waitumbi J, Warburg A. Inf Immun. 1998;66:1534–1537. doi: 10.1128/iai.66.4.1534-1537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]