Abstract
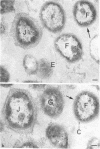
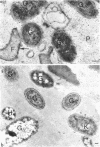
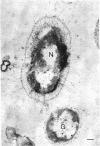
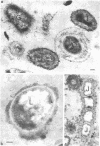
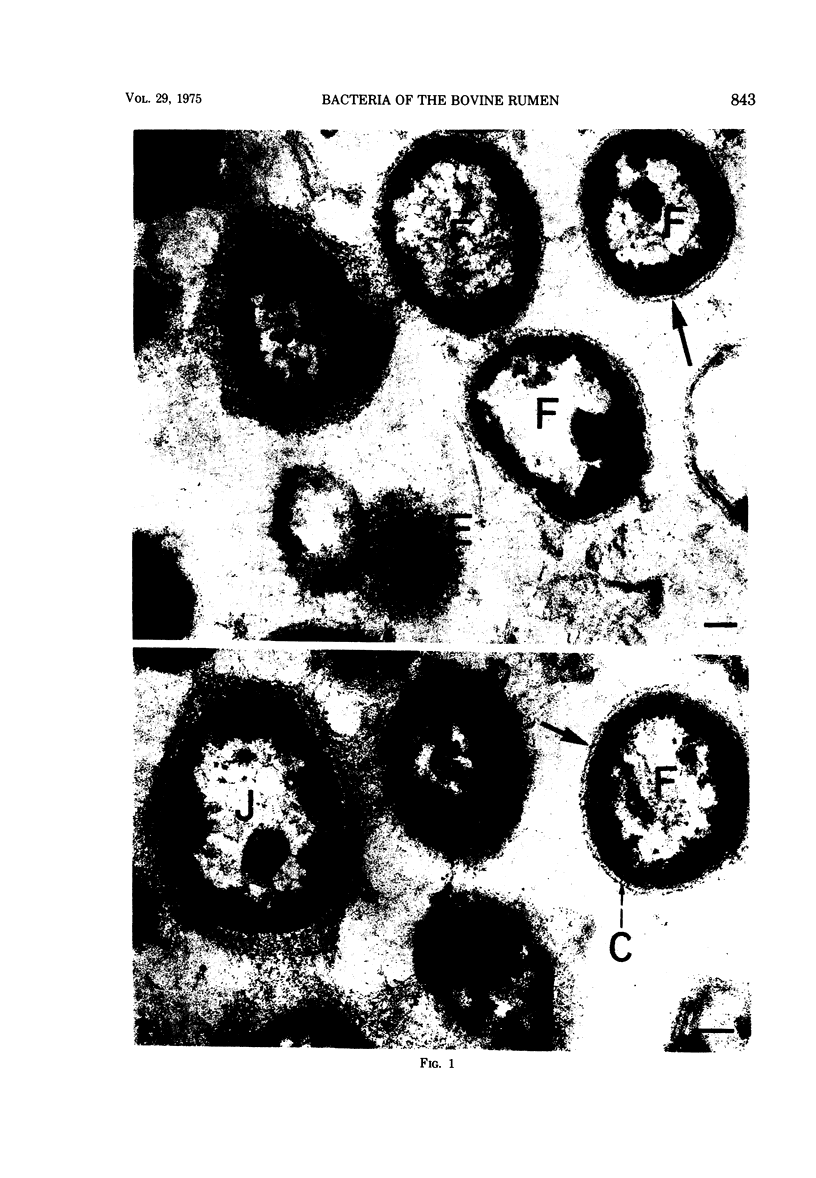
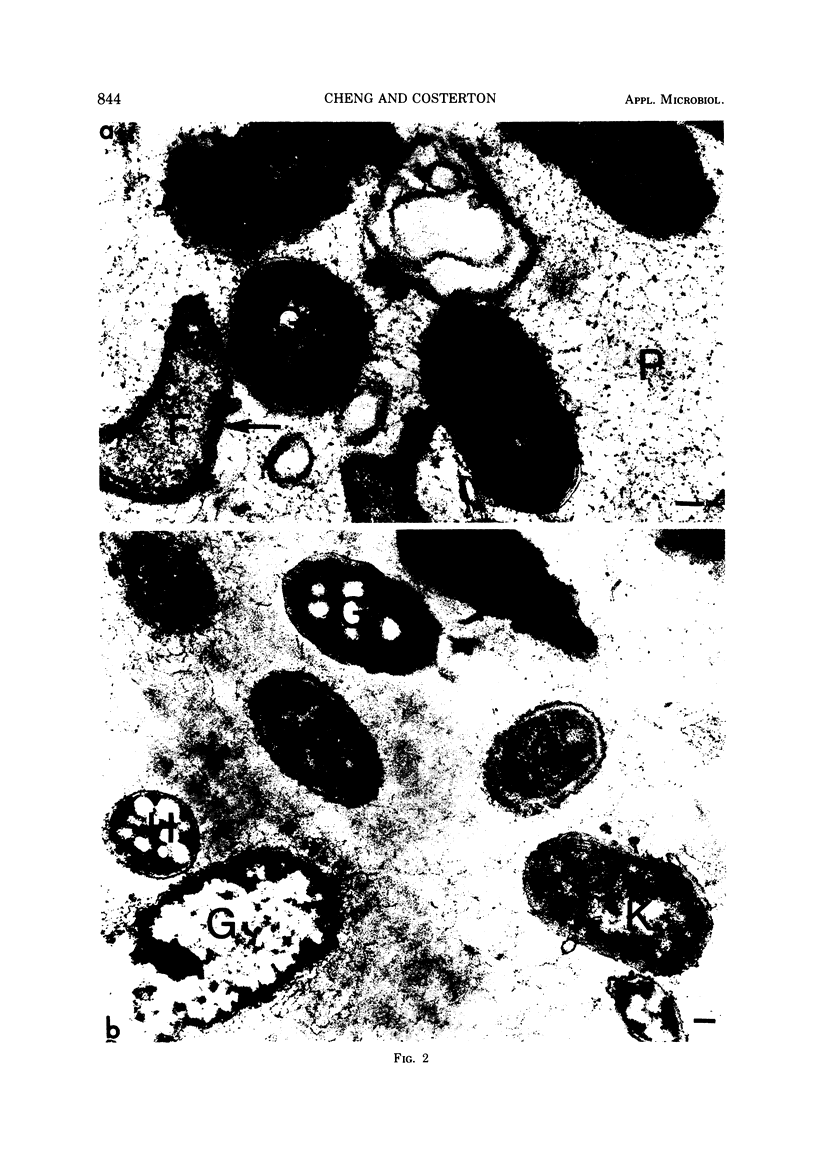
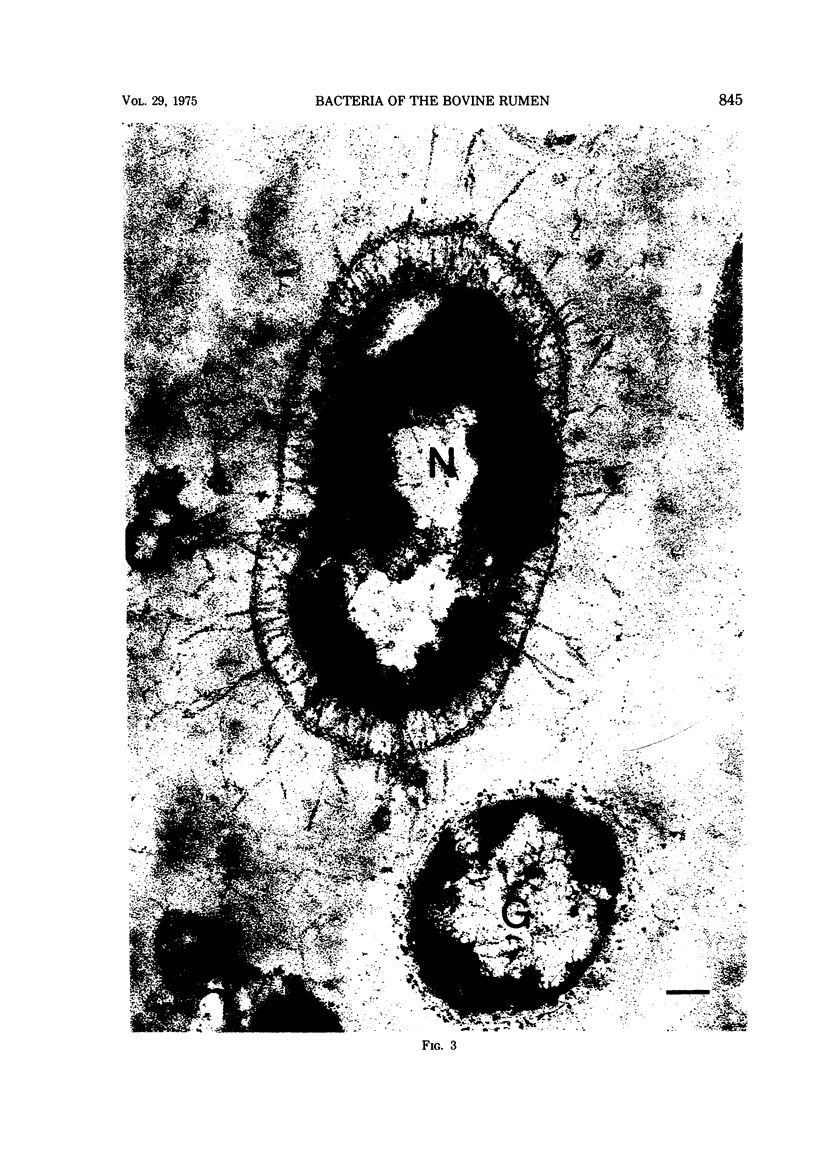
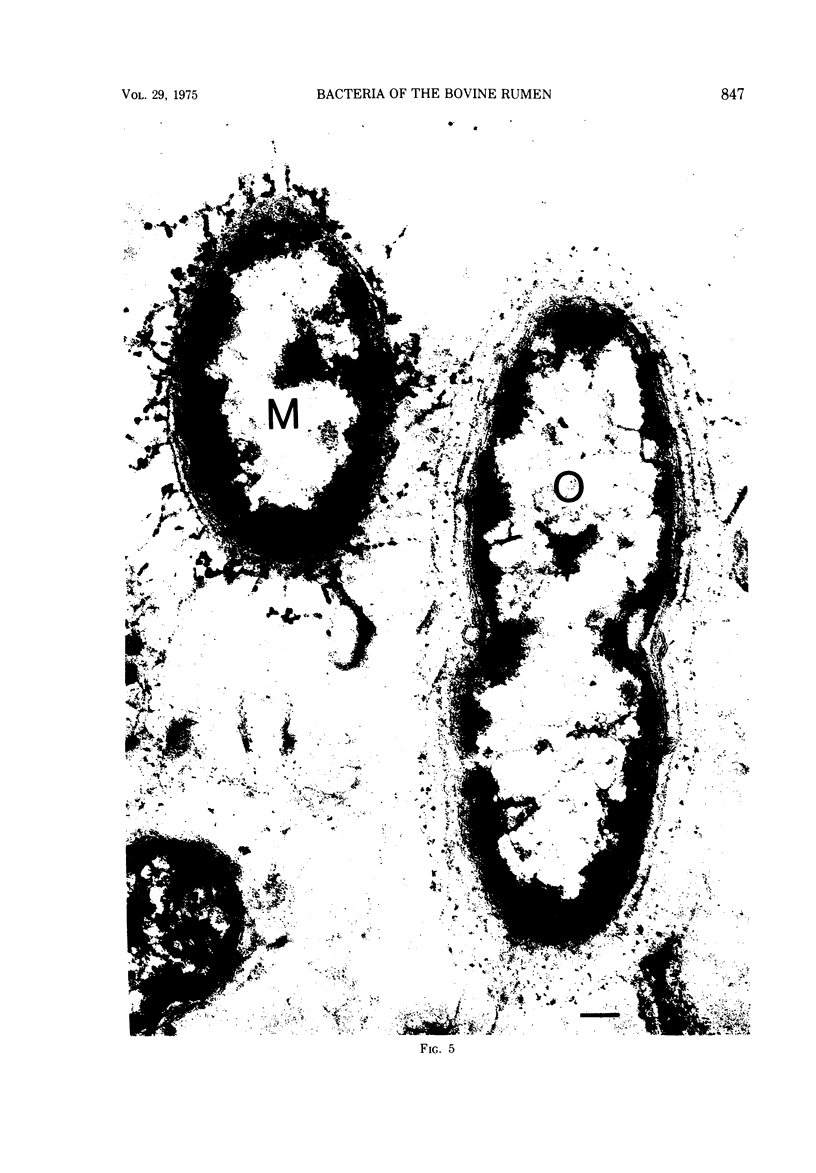
Most of the bacteria found in rumen fluid samples taken from cows fed hay, or a concentrate diet, had cell walls of the gram-negative type. Most were intact, with only a small proportion of lysed cells, and many of the cells contained electron-translucent cytoplasmic deposits similar to the carbohydrate reserve material described in pure cultures of rumen organisms. All of the bacteria observed in these samples had an external “coat” layer outside the outer membrane when fixed in glutaraldehyde and osmium, stained with uranyl acetate and lead citrate, and examined as sectioned material. These coat layers varied from thin (ca. 8 nm) structures to very extensive fibrous systems, sometimes including concentric arrangements and radial fibers extending up to 1,200 nm from the cell. The thin-coat layers sometimes exhibited a rough periodicity. In all, 10 different types of coat layers were distinguished on a morphological basis. It is proposed that these external coat layers have protective and adherence functions for the rumen bacteria in the environment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. C., Casida L. E., Jr Responses of indigenous microorganisms to soil incubation as viewed by transmission electron microscopy of cell thin sections. J Bacteriol. 1973 Mar;113(3):1462–1473. doi: 10.1128/jb.113.3.1462-1473.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Casida L. E., Jr Microflora of soil as viewed by freeze-etching. J Bacteriol. 1973 Jun;114(3):1319–1327. doi: 10.1128/jb.114.3.1319-1327.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- COSTERTON J. W., MURRAY R. G., ROBINOW C. F. Observations on the motility and the structure of Vitreoscilla. Can J Microbiol. 1961 Jun;7:329–339. doi: 10.1139/m61-040. [DOI] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S., Howard B. H. Ultrastructural studies on Selenomonas ruminantium from the sheep rumen. J Gen Microbiol. 1973 Nov;79(1):135–146. doi: 10.1099/00221287-79-1-135. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Roberts D. W., Costerton J. W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can J Microbiol. 1973 Dec;19(12):1501–1506. doi: 10.1139/m73-244. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkinson J. F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969 Dec;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Cheng K. J. Effect of the removal of outer cell wall layers on the actinomycin susceptibility of a gram-negative bacterium. Antimicrob Agents Chemother. 1972 May;1(5):447–449. doi: 10.1128/aac.1.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Protective role of smooth lipopolysaccharide in the serum bactericidal reaction. Infect Immun. 1971 Dec;4(6):764–771. doi: 10.1128/iai.4.6.764-771.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. M., MacLeod R. A. Factors affecting the activity and stability of alkaline phosphatase in a marine pseudomonad. J Bacteriol. 1974 Feb;117(2):813–818. doi: 10.1128/jb.117.2.813-818.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Macromolecular subunits in the walls of marine nitrifying bacteria. Science. 1969 Feb 14;163(3868):685–686. doi: 10.1126/science.163.3868.685. [DOI] [PubMed] [Google Scholar]
- Work E., Knox K. W., Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):438–449. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]