Abstract
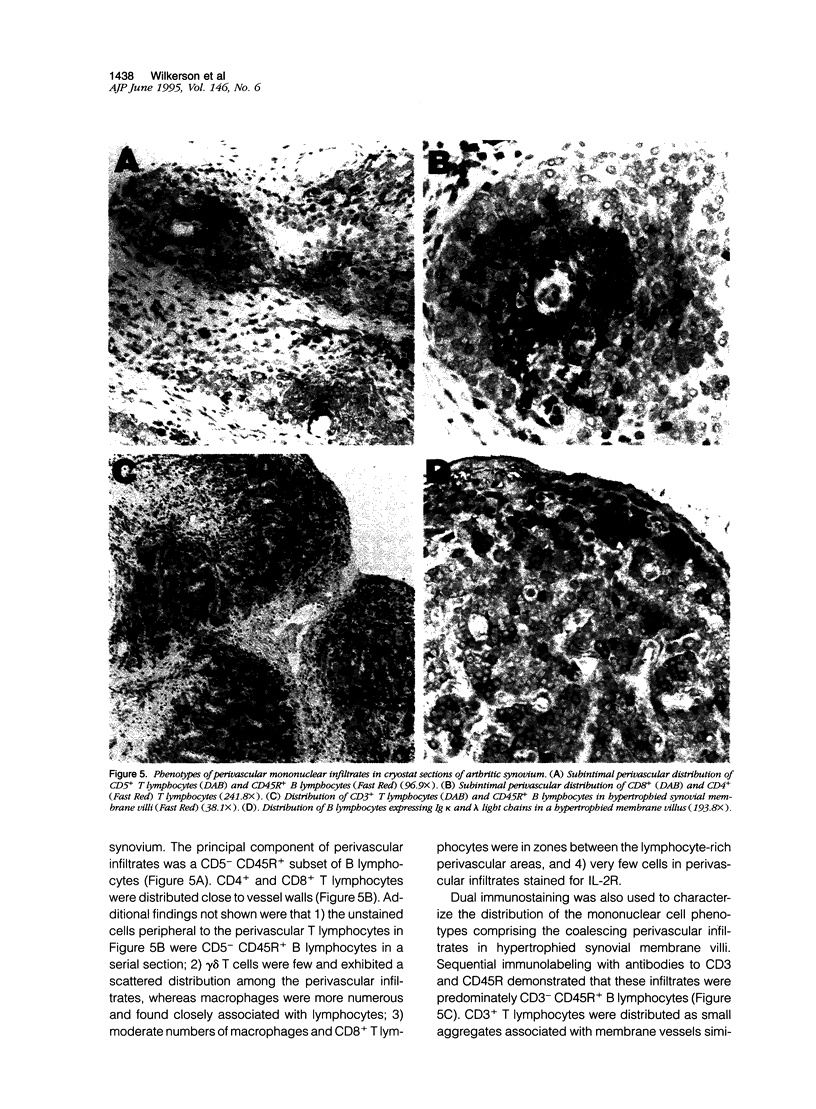
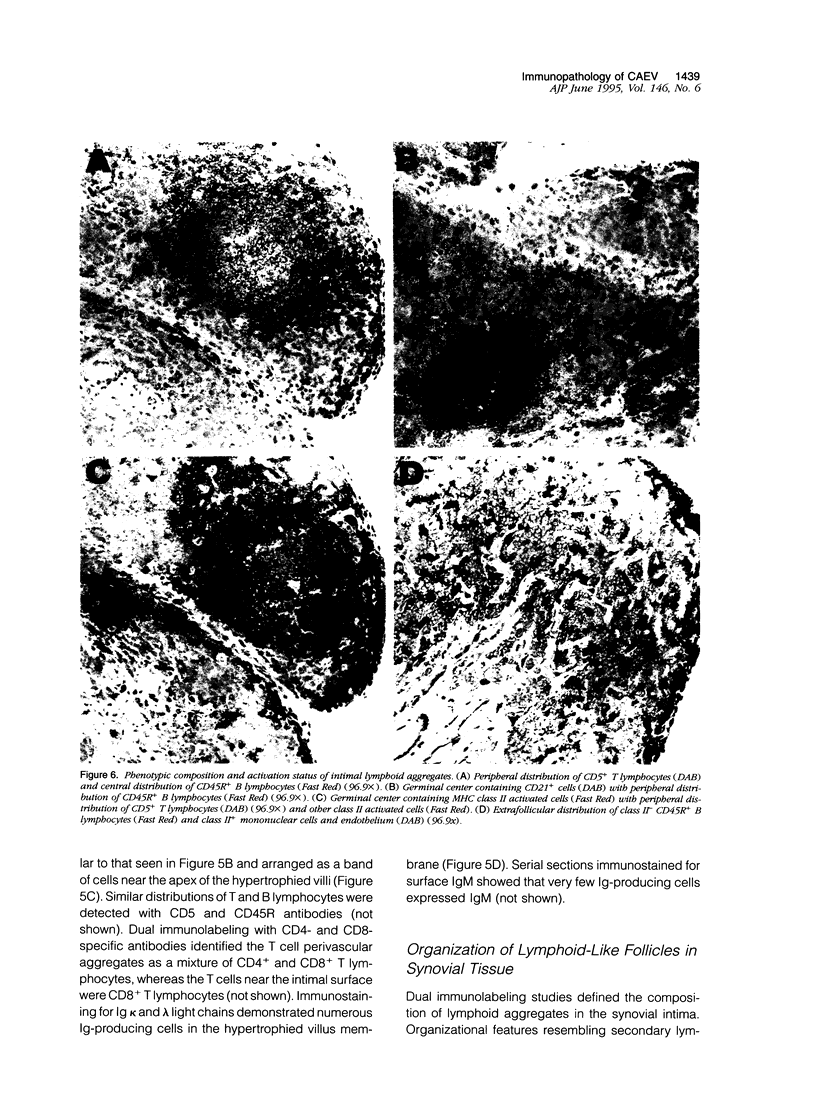
This study evaluated histopathology and mononuclear cell phenotypes in synovial lesions of chronic arthritis induced by experimental infection of Saanen goats with caprine arthritis-encephalitis lentivirus. Histological examination of carpal joint synovium of three infected goats with clinical arthritis revealed progressive lesions consisting of membrane villus hypertrophy with extensive angiogenesis and mononuclear cell infiltration and degenerative changes of membrane villus necrosis associated with loss of vasculature and infiltrates. Changes in synovial tissue of five age-matched infected goats without clinical arthritis were limited to moderate synovial membrane hyperplasia also noted in an age-matched uninfected goat. Immunohistochemistry identified CD45R+ CD5- B lymphocytes as the principal component of most perivascular infiltrates in arthritic synovium. Other mononuclear cells included perivascular CD4+ and CD8+ T lymphocytes and macrophages with a prominent accumulation of CD8+ T lymphocytes at the lining surface of inflamed villi. T lymphocytes and macrophages as well as synovial lining cells were activated with respect to MHC class II but not for interleukin-2 receptors. Inflamed villi also contained lymphoid aggregates comprised of B cell germinal centers and activated T-cell mantles. B cells expressing immunoglobulin occurred around follicles and throughout inflamed villi. These findings indicate that memory immune responses that favor expansion and maturation of B cells and immunoglobulin production contribute to the immunopathology of chronic arthritis.
Full text
PDF


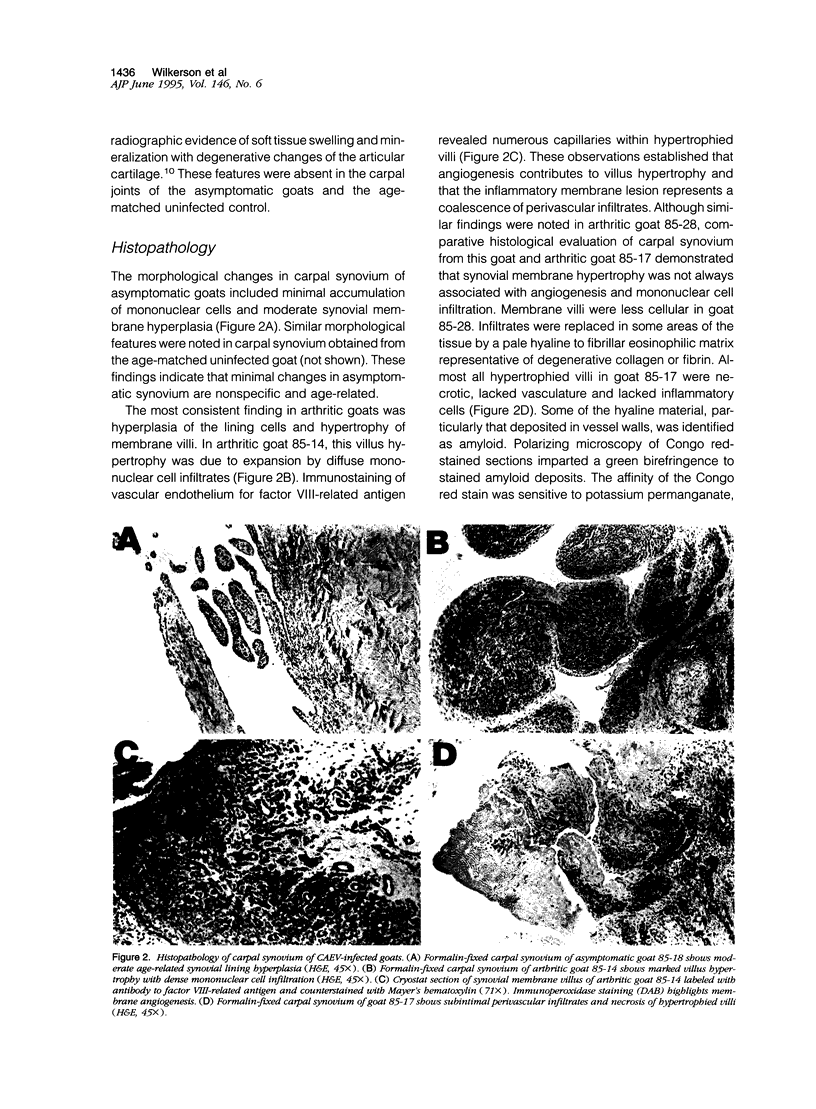




Images in this article
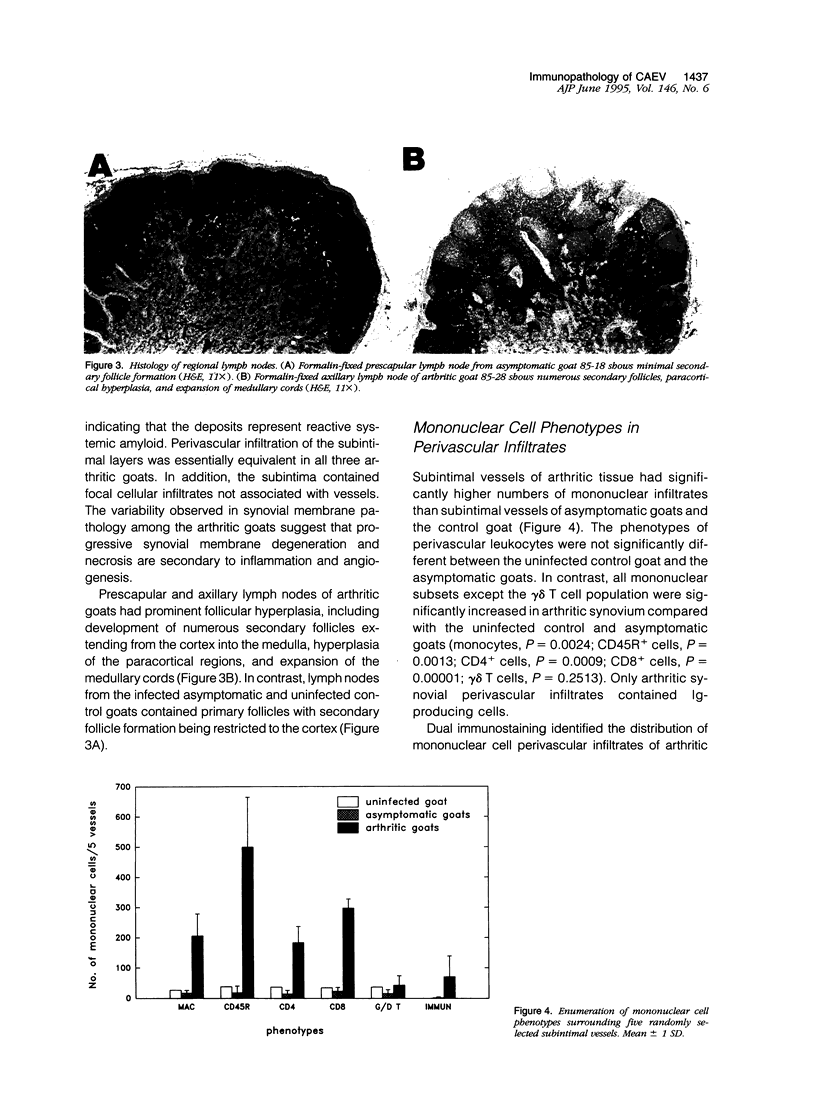
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ababou A., Davis W. C., Levy D. The DA6-147 monoclonal antibody raised against the HLA-DR alpha chain identifies a cryptic epitope on the BoLA-DR alpha chain. Vet Res. 1993;24(5):402–407. [PubMed] [Google Scholar]
- Aydintug M. K., Inzana T. J., Letonja T., Davis W. C., Corbeil L. B. Cross-reactivity of monoclonal antibodies to Escherichia coli J5 with heterologous gram-negative bacteria and extracted lipopolysaccharides. J Infect Dis. 1989 Nov;160(5):846–857. doi: 10.1093/infdis/160.5.846. [DOI] [PubMed] [Google Scholar]
- Baszler T. V., Zachary J. F. Murine retroviral neurovirulence correlates with an enhanced ability ofvirus to infect selectively, replicate in, and activate resident microglial cells. Am J Pathol. 1991 Mar;138(3):655–671. [PMC free article] [PubMed] [Google Scholar]
- Bembridge G. P., Parsons K. R., Sopp P., MacHugh N. D., Howard C. J. Comparison of monoclonal antibodies with potential specificity for restricted isoforms of the leukocyte common antigen (CD45R). Vet Immunol Immunopathol. 1993 Nov;39(1-3):129–136. doi: 10.1016/0165-2427(93)90173-2. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Knowles D. P., Jr, Norton L. K. Neutralization-resistant antigenic variants of caprine arthritis-encephalitis lentivirus associated with progressive arthritis. J Infect Dis. 1991 Oct;164(4):679–685. doi: 10.1093/infdis/164.4.679. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Knowles D. P., McGuire T. C., Cunningham D. R., Adams D. S., Gorham J. R. Chronic disease in goats orally infected with two isolates of the caprine arthritis-encephalitis lentivirus. Lab Invest. 1988 May;58(5):510–517. [PubMed] [Google Scholar]
- Cheevers W. P., McGuire T. C. The lentiviruses: maedi/visna, caprine arthritis-encephalitis, and equine infectious anemia. Adv Virus Res. 1988;34:189–215. doi: 10.1016/s0065-3527(08)60518-7. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Invest. 1980 Jun;42(6):596–602. [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Sande R. D., Gorham J. R., Henson J. B. The connective tissue component of the caprine arthritis-encephalitis syndrome. Am J Pathol. 1980 Aug;100(2):443–454. [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Cellular basis for rheumatoid inflammation. Clin Orthop Relat Res. 1991 Apr;(265):9–22. [PubMed] [Google Scholar]
- Davis W. C., Ellis J. A. Individual antigens of goats. Vet Immunol Immunopathol. 1991 Jan;27(1-3):121–131. doi: 10.1016/0165-2427(91)90091-p. [DOI] [PubMed] [Google Scholar]
- Davis W. C., Marusic S., Lewin H. A., Splitter G. A., Perryman L. E., McGuire T. C., Gorham J. R. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet Immunol Immunopathol. 1987 Jul;15(4):337–376. doi: 10.1016/0165-2427(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Lees A., Morris S. C. Antigen presentation by B lymphocytes to CD4+ T lymphocytes in vivo: importance for B lymphocyte and T lymphocyte activation. Semin Immunol. 1992 Aug;4(4):247–255. [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Gogolewski R. P., Adams D. S., McGuire T. C., Banks K. L., Cheevers W. P. Antigenic cross-reactivity between caprine arthritis-encephalitis, visna and progressive pneumonia viruses involves all virion-associated proteins and glycoproteins. J Gen Virol. 1985 Jun;66(Pt 6):1233–1240. doi: 10.1099/0022-1317-66-6-1233. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Jackson M. K., Knowles D. P., Stem T. A., Harwood W. G., Robinson M. M., Cheevers W. P. Genetic structure of the pol-env region of the caprine arthritis-encephalitis lentivirus genome. Virology. 1991 Jan;180(1):389–394. doi: 10.1016/0042-6822(91)90044-c. [DOI] [PubMed] [Google Scholar]
- Johnson G. C., Adams D. S., McGuire T. C. Pronounced production of polyclonal immunoglobulin G1 in the synovial fluid of goats with caprine arthritis-encephalitis virus infection. Infect Immun. 1983 Aug;41(2):805–815. doi: 10.1128/iai.41.2.805-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Zink C., Narayan O. Pathogenesis of ovine lentivirus-induced arthritis: phenotypic evaluation of T lymphocytes in synovial fluid, synovium, and peripheral circulation. Clin Immunol Immunopathol. 1989 Aug;52(2):323–330. doi: 10.1016/0090-1229(89)90183-9. [DOI] [PubMed] [Google Scholar]
- Knowles D. P., Jr, Cheevers W. P., McGuire T. C., Brassfield A. L., Harwood W. G., Stem T. A. Structure and genetic variability of envelope glycoproteins of two antigenic variants of caprine arthritis-encephalitis lentivirus. J Virol. 1991 Nov;65(11):5744–5750. doi: 10.1128/jvi.65.11.5744-5750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D., Jr, Cheevers W., McGuire T., Stem T., Gorham J. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J Virol. 1990 May;64(5):2396–2398. doi: 10.1128/jvi.64.5.2396-2398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Ziesmer S. C., Lazcano-Villareal O. Use of azide and hydrogen peroxide as an inhibitor for endogenous peroxidase in the immunoperoxidase method. J Histochem Cytochem. 1987 Dec;35(12):1457–1460. doi: 10.1177/35.12.2824601. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Mandler R., Chu C. C., Paul W. E., Max E. E., Snapper C. M. Interleukin 5 induces S mu-S gamma 1 DNA rearrangement in B cells activated with dextran-anti-IgD antibodies and interleukin 4: a three component model for Ig class switching. J Exp Med. 1993 Nov 1;178(5):1577–1586. doi: 10.1084/jem.178.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Brassfield A. L., Davis W. C., Cheevers W. P. Antigenic and structural variation of the p28 core polypeptide of goat and sheep retroviruses. J Gen Virol. 1987 Aug;68(Pt 8):2259–2263. doi: 10.1099/0022-1317-68-8-2259. [DOI] [PubMed] [Google Scholar]
- Morikawa K., Oseko F., Morikawa S. The role of CD45RA on human B-cell function: anti-CD45RA antibody (anti-2H4) inhibits the activation of resting B cells and antibody production of activated B cells independently in humans. Scand J Immunol. 1991 Sep;34(3):273–283. doi: 10.1111/j.1365-3083.1991.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Naessens J., Sileghem M., MacHugh N., Park Y. H., Davis W. C., Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology. 1992 Jun;76(2):305–309. [PMC free article] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Parker D. C. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I., Frizziero L., Guerra D., Baccarani-Contri M., Focherini M. C., Georgountzos A., Vincenzi D., Cicchetti F., Perbellini A., Govoni E. Aging of the human synovium: an in vivo and ex vivo morphological study. Semin Arthritis Rheum. 1992 Jun;21(6):400–414. doi: 10.1016/0049-0172(92)90041-b. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Wilkerson M. J., Hullinger G. A., Cheevers W. P. Depressed CD4+ T lymphocyte proliferative response and enhanced antibody response to viral antigen in chronic lentivirus-induced arthritis. J Infect Dis. 1995 Feb;171(2):328–334. doi: 10.1093/infdis/171.2.328. [DOI] [PubMed] [Google Scholar]
- Rizzo L. V., DeKruyff R. H., Umetsu D. T. Generation of B cell memory and affinity maturation. Induction with Th1 and Th2 T cell clones. J Immunol. 1992 Jun 15;148(12):3733–3739. [PubMed] [Google Scholar]
- Smith M. D., O'Donnell J., Highton J., Palmer D. G., Rozenbilds M., Roberts-Thomson P. J. Immunohistochemical analysis of synovial membranes from inflammatory and non-inflammatory arthritides: scarcity of CD5 positive B cells and IL2 receptor bearing T cells. Pathology. 1992 Jan;24(1):19–26. doi: 10.3109/00313029209063615. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Kapasi Z. F., Masuda A., Tew J. G. Follicular dendritic cells in the alternative antigen transport pathway: microenvironment, cellular events, age and retrovirus related alterations. Semin Immunol. 1992 Aug;4(4):257–265. [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Expression of C3d receptors during human B cell differentiation: immunofluorescence analysis with the HB-5 monoclonal antibody. J Immunol. 1984 Aug;133(2):678–683. [PubMed] [Google Scholar]
- Terashima K., Dobashi M., Maeda K., Imai Y. Follicular dendritic cell and ICCOSOMES in germinal center reactions. Semin Immunol. 1992 Aug;4(4):267–274. [PubMed] [Google Scholar]
- Tew J. G., Kosco M. H., Szakal A. K. The alternative antigen pathway. Immunol Today. 1989 Jul;10(7):229–232. doi: 10.1016/0167-5699(89)90258-2. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Wilkerson M. J., Davis W. C., Cheevers W. P. Peripheral blood and synovial fluid mononuclear cell phenotypes in lentivirus induced arthritis. J Rheumatol. 1995 Jan;22(1):8–15. [PubMed] [Google Scholar]
- Woodard J. C., Gaskin J. M., Poulos P. W., MacKay R. J., Burridge M. J. Caprine arthritis-encephalitis: clinicopathologic study. Am J Vet Res. 1982 Dec;43(12):2085–2096. [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Bresnihan B. Analysis of cell populations in rheumatoid arthritis synovial tissues. Semin Arthritis Rheum. 1992 Jun;21(6):393–399. doi: 10.1016/0049-0172(92)90040-k. [DOI] [PubMed] [Google Scholar]
- el-Gabalawy H. S., Keillor J. Immunohistologic study of T-cell receptor delta-chain expression in rheumatoid synovial membranes. Semin Arthritis Rheum. 1992 Feb;21(4):239–245. doi: 10.1016/0049-0172(92)90054-h. [DOI] [PubMed] [Google Scholar]