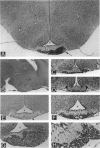
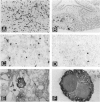
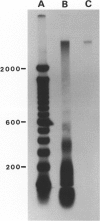
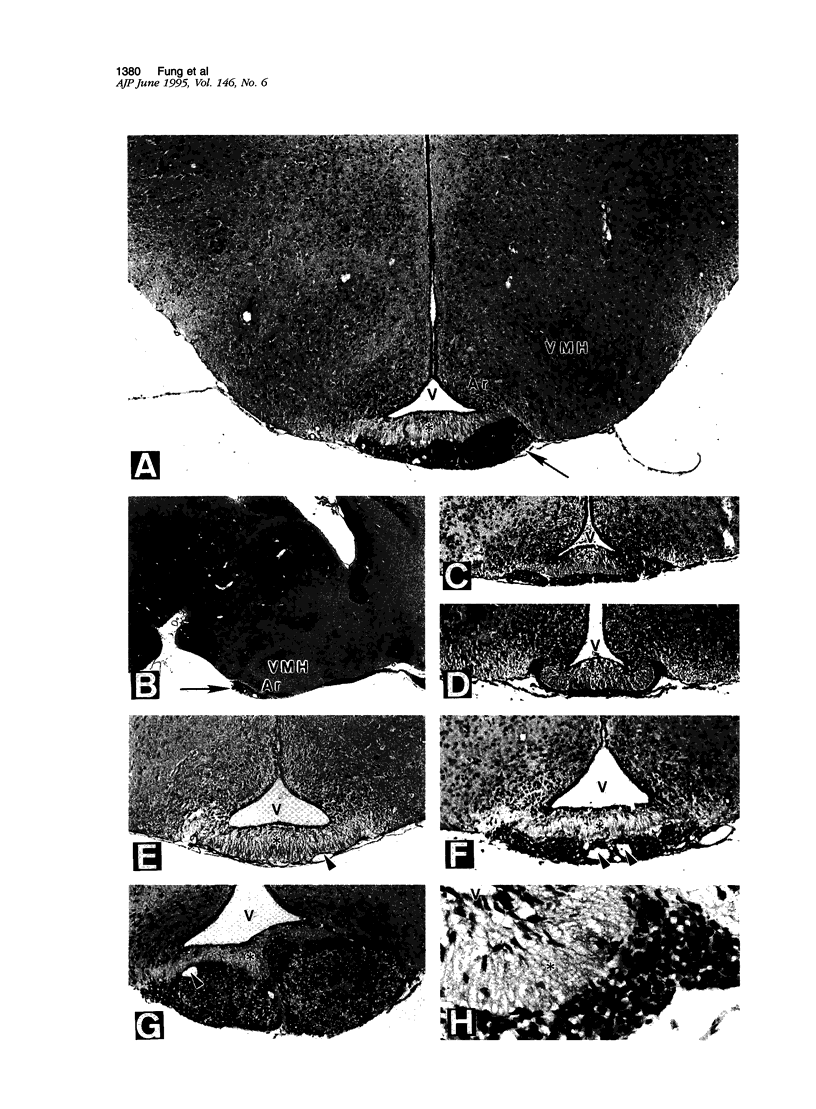
Abstract
Cell proliferation and cell death play critical roles in embryonic development, postnatal tissue maintenance, and tumor formation. To understand the interplay between cell proliferation and death in tumor formation, we studied these two processes in nascent primitive neuroectodermal tumors that arose postnatally from neuroepithelial cells ventral to the median eminence of transgenic mice (designated rTH-Tag mice) carrying a Simian virus 40 large T antigen transgene driven by a rat tyrosine hydroxylase promoter. Cell proliferation continued in the neuroepithelium of the ventral median eminence in wild-type and transgenic animals for the first 2 weeks of postnatal life but subsided completely in the wild-type mice after 2 weeks of age. In contrast, mitotic activity persisted in these progenitor cells of the rTH-Tag mice, and there was a dramatic increase in mitotic activity after 10 weeks leading to the formation of primitive neuroectodermal tumors despite sustained cell death activity. We conclude that primitive neuroectodermal tumors originate from progenitor cells in the ventral median eminence of rTH-Tag mice in early postnatal life when progenitors fail to respond to signals to exit the cell cycle. Thus, the disruption of mechanisms that regulate cell proliferation and cell death in the developing brain may underlie the emergence of primitive neuroectodermal tumors in the rTH-Tag mice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Arends M. J., McGregor A. H., Wyllie A. H. Apoptosis is inversely related to necrosis and determines net growth in tumors bearing constitutively expressed myc, ras, and HPV oncogenes. Am J Pathol. 1994 May;144(5):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Boniuk M., Girard L. J. Spontaneous regression of bilateral retinoblastoma. Trans Am Acad Ophthalmol Otolaryngol. 1969 Mar-Apr;73(2):194–198. [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen N. L. Neuroblastoma: epidemiology and pattern of regression. Problems in interpreting results of mass screening. Am J Pediatr Hematol Oncol. 1992 May;14(2):103–110. [PubMed] [Google Scholar]
- Ceballos P. I., Barnhill R. L. Spontaneous regression of cutaneous tumors. Adv Dermatol. 1993;8:229–262. [PubMed] [Google Scholar]
- Christofori G., Naik P., Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994 Jun 2;369(6479):414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- Efrat S., Teitelman G., Anwar M., Ruggiero D., Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic alpha cells. Neuron. 1988 Sep;1(7):605–613. doi: 10.1016/0896-6273(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39(2):451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Estus S., Johnson E. M., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994 Feb;12(2):343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Fukasawa Y., Ishikura H., Takada A., Yokoyama S., Imamura M., Yoshiki T., Sato H. Massive apoptosis in infantile myofibromatosis. A putative mechanism of tumor regression. Am J Pathol. 1994 Mar;144(3):480–485. [PMC free article] [PubMed] [Google Scholar]
- Fung K. M., Chikaraishi D. M., Suri C., Theuring F., Messing A., Albert D. M., Lee V. M., Trojanowski J. Q. Molecular phenotype of simian virus 40 large T antigen-induced primitive neuroectodermal tumors in four different lines of transgenic mice. Lab Invest. 1994 Jan;70(1):114–124. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Jansson D. S., Molenaar W. M., Rorke L. B., Trojanowski J. Q., Lee V. M., Packer R. J., Franke W. W. Primitive neuroectodermal tumors of the central nervous system. Patterns of expression of neuroendocrine markers, and all classes of intermediate filament proteins. Lab Invest. 1990 Apr;62(4):498–509. [PubMed] [Google Scholar]
- Hammang J. P., Behringer R. R., Baetge E. E., Palmiter R. D., Brinster R. L., Messing A. Oncogene expression in retinal horizontal cells of transgenic mice results in a cascade of neurodegeneration. Neuron. 1993 Jun;10(6):1197–1209. doi: 10.1016/0896-6273(93)90067-2. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Earle K. M. Primitive neuroectodermal tumors of the brain in children. Cancer. 1973 Oct;32(4):890–897. doi: 10.1002/1097-0142(197310)32:4<890::aid-cncr2820320421>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hermeking H., Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994 Sep 30;265(5181):2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent B. L., Sosnowski R. G., Reed R. R. Directed expression of an oncogene to the olfactory neuronal lineage in transgenic mice. J Neurosci. 1993 Jan;13(1):300–312. doi: 10.1523/JNEUROSCI.13-01-00300.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini L., Del Vecchio M. T., Megha T., Barbini P., Galieni P., Pileri S., Sabattini E., Gherlinzoni F., Tosi P., Kraft R. Correlations between apoptotic and proliferative indices in malignant non-Hodgkin's lymphomas. Am J Pathol. 1993 Mar;142(3):755–763. [PMC free article] [PubMed] [Google Scholar]
- Merlino G. Regulatory imbalances in cell proliferation and cell death during oncogenesis in transgenic mice. Semin Cancer Biol. 1994 Feb;5(1):13–20. [PubMed] [Google Scholar]
- Meyer J. S., Nauert J., Koehm S., Hughes J. Cell kinetics of human tumors by in vitro bromodeoxyuridine labeling. J Histochem Cytochem. 1989 Sep;37(9):1449–1454. doi: 10.1177/37.9.2768814. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Molenaar W. M., Jansson D. S., Gould V. E., Rorke L. B., Franke W. W., Lee V. M., Packer R. J., Trojanowski J. Q. Molecular markers of primitive neuroectodermal tumors and other pediatric central nervous system tumors. Monoclonal antibodies to neuronal and glial antigens distinguish subsets of primitive neuroectodermal tumors. Lab Invest. 1989 Dec;61(6):635–643. [PubMed] [Google Scholar]
- Monroe B. G., Paull W. K. Ultrastructural changes in the hypothalamus during development and hypothalamic activity: the median eminence. Prog Brain Res. 1974;41:185–208. doi: 10.1016/S0079-6123(08)61907-X. [DOI] [PubMed] [Google Scholar]
- Nowakowski R. S., Lewin S. B., Miller M. W. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989 Jun;18(3):311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Pan H., Griep A. E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994 Jun 1;8(11):1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Acheson A. L., Thibault J., Thoenen H. Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J Neurosci. 1986 Sep;6(9):2616–2624. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorke L. B. Experimental production of primitive neuroectodermal tumors and its relevance to human neuro-oncology. Am J Pathol. 1994 Mar;144(3):444–448. [PMC free article] [PubMed] [Google Scholar]
- Rorke L. B. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 1983 Jan;42(1):1–15. [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Schiffer D., Cavalla P., Chiò A., Giordana M. T., Marino S., Mauro A., Migheli A. Tumor cell proliferation and apoptosis in medulloblastoma. Acta Neuropathol. 1994;87(4):362–370. doi: 10.1007/BF00313605. [DOI] [PubMed] [Google Scholar]
- Sepulveda A. R., Finegold M. J., Smith B., Slagle B. L., DeMayo J. L., Shen R. F., Woo S. L., Butel J. S. Development of a transgenic mouse system for the analysis of stages in liver carcinogenesis using tissue-specific expression of SV40 large T-antigen controlled by regulatory elements of the human alpha-1-antitrypsin gene. Cancer Res. 1989 Nov 1;49(21):6108–6117. [PubMed] [Google Scholar]
- Suri C., Fung B. P., Tischler A. S., Chikaraishi D. M. Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigen transgenic mice. J Neurosci. 1993 Mar;13(3):1280–1291. doi: 10.1523/JNEUROSCI.13-03-01280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Ishihara M., Kitagawa M., Harada H., Kimura T., Matsuyama T., Lamphier M. S., Aizawa S., Mak T. W., Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994 Jun 17;77(6):829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Tilly J. L., Hsueh A. J. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993 Mar;154(3):519–526. doi: 10.1002/jcp.1041540310. [DOI] [PubMed] [Google Scholar]
- Tohyama T., Lee V. M., Rorke L. B., Marvin M., McKay R. D., Trojanowski J. Q. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992 Mar;66(3):303–313. [PubMed] [Google Scholar]
- Trojanowski J. Q., Fung K. M., Rorke L. B., Tohyama T., Yachnis A. T., Lee V. M. In vivo and in vitro models of medulloblastomas and other primitive neuroectodermal brain tumors of childhood. Mol Chem Neuropathol. 1994 Feb-Apr;21(2-3):219–239. doi: 10.1007/BF02815352. [DOI] [PubMed] [Google Scholar]
- Ugrumov M. V. Development of the median eminence during ontogenesis (morpho-functional aspects). Prog Brain Res. 1992;91:349–356. doi: 10.1016/s0079-6123(08)62353-5. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- White K., Grether M. E., Abrams J. M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994 Apr 29;264(5159):677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993 Feb;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis A. T., Rorke L. B., Lee V. M., Trojanowski J. Q. Expression of neuronal and glial polypeptides during histogenesis of the human cerebellar cortex including observations on the dentate nucleus. J Comp Neurol. 1993 Aug 15;334(3):356–369. doi: 10.1002/cne.903340303. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Rorke L. B., Trojanowski J. Q. Cerebellar dysplasias in humans: development and possible relationship to glial and primitive neuroectodermal tumors of the cerebellar vermis. J Neuropathol Exp Neurol. 1994 Jan;53(1):61–71. doi: 10.1097/00005072-199401000-00008. [DOI] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Hollyfield J. G., Overbeek P. A., Baehr W. Photoreceptor degeneration induced by the expression of simian virus 40 large tumor antigen in the retina of transgenic mice. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1194–1198. doi: 10.1073/pnas.89.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]