Abstract
Mesenchymal tumors of the lower reproductive tract of women are poorly understood at the molecular level as a result in part of the lack of relevant animal models. The present study describes a novel model of gynecological smooth muscle tumors in which these neoplasms arise in Eker rats as part of a familial cancer syndrome. The tumors develop as a result of a germline mutation in the tuberous sclerosis 2 (TSC2) gene, and predisposition to tumor development is inherited in an autosomal dominant fashion. Uterine and/or cervical tumors arise spontaneously as single or multicentric neoplasms and increase in incidence with increasing age. The tumors were classified into three phenotypic variants of leiomyoma/leiomyosarcoma and into stromal cervicovaginal tumors on the basis of cytological and histological features and immunostaining patterns for smooth muscle actin and desmin. Tumors histologically identical to the typical human myometrial leiomyoma arose, as did a subset of atypical leiomyomas having an epithelioid phenotype. Eker rats were found to develop both benign and malignant smooth muscle tumors. The high spontaneous incidence of smooth muscle tumors of uterus and cervix in this rodent model provides a unique opportunity to study the molecular mechanisms underlying the development of these clinically important gynecological neoplasms.
Full text
PDF




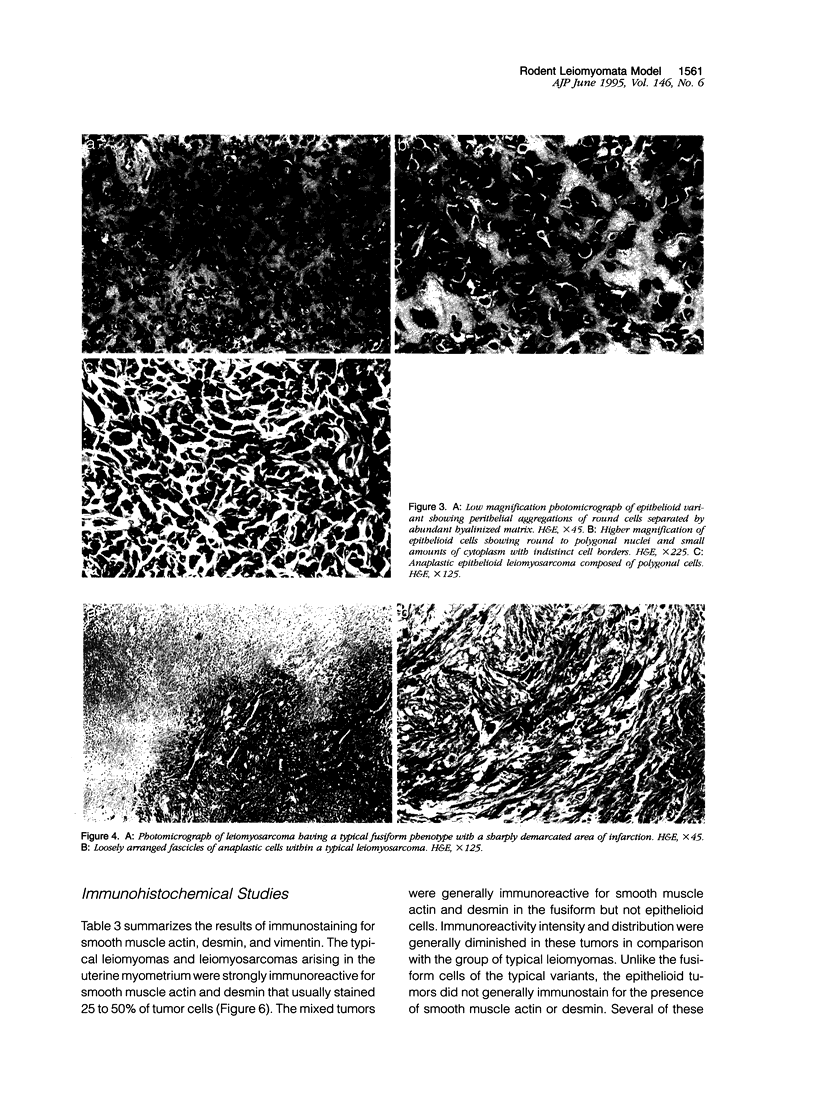
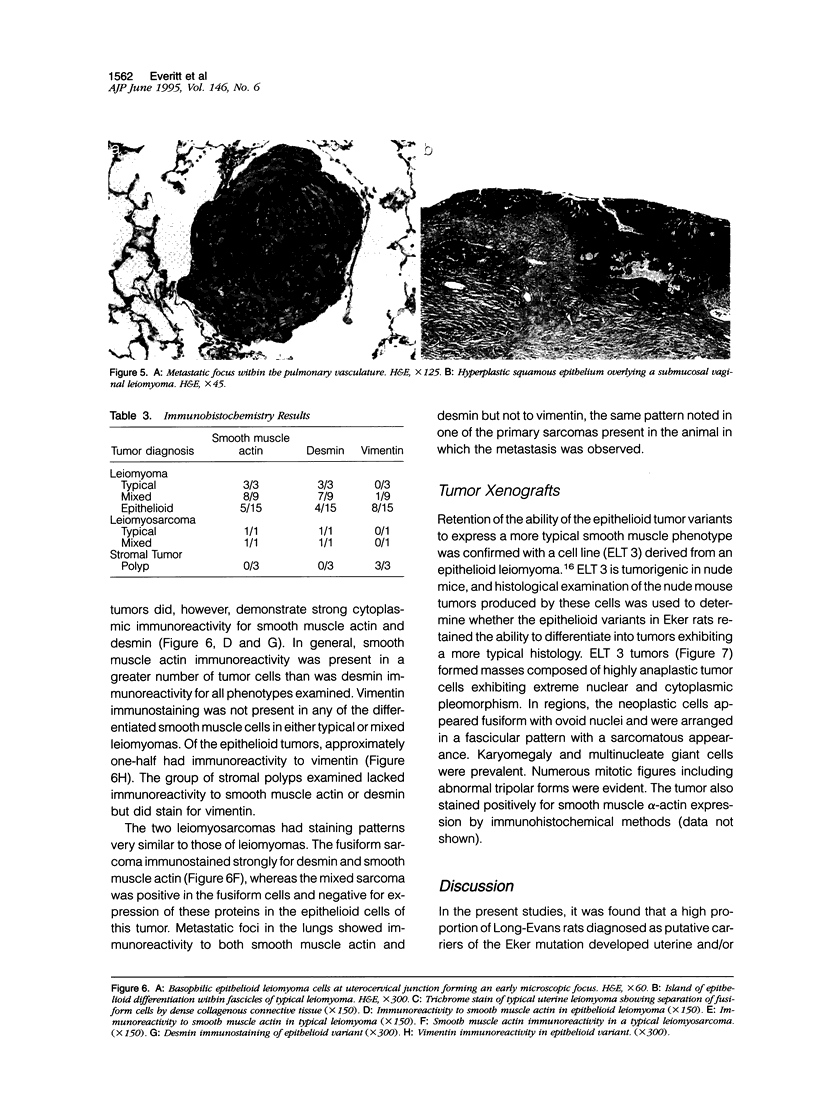
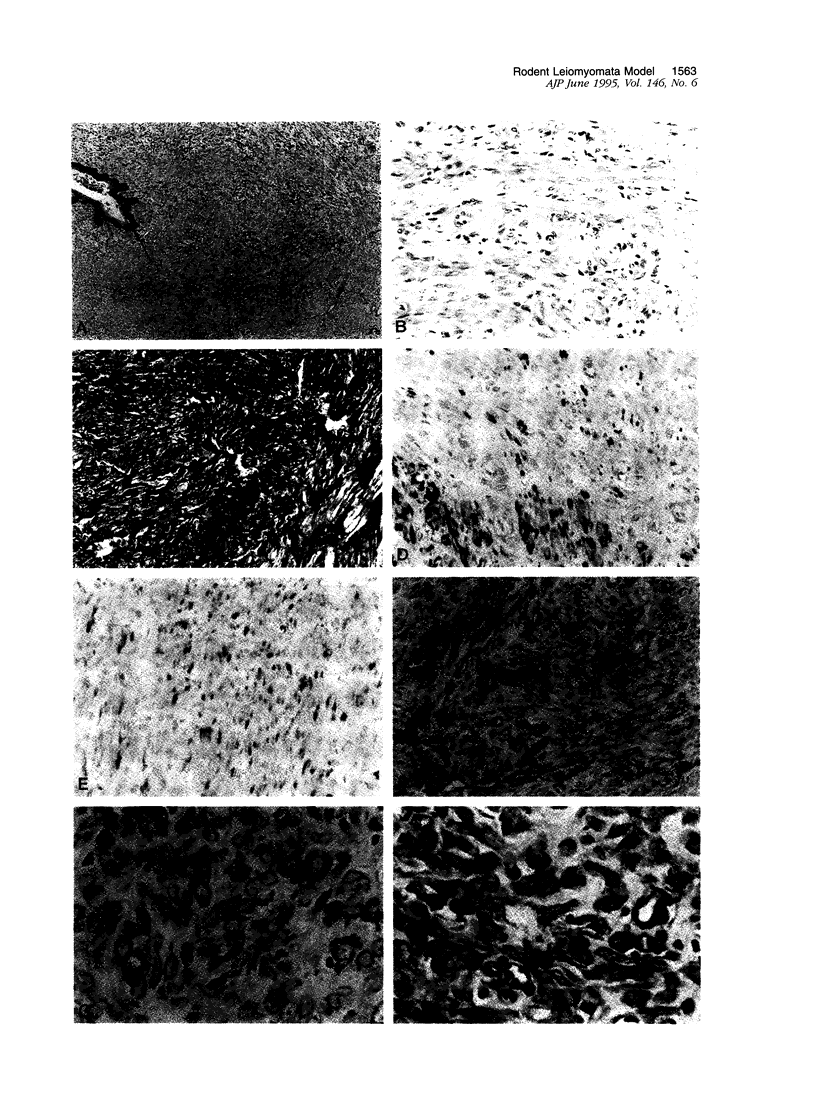




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burek J. D., Hollander C. F. High incidence of spontaneous cervical and vaginal tumors in an inbred strain of Brown Norway rats (BN/Bi). J Natl Cancer Inst. 1976 Sep;57(3):549–554. doi: 10.1093/jnci/57.3.549. [DOI] [PubMed] [Google Scholar]
- Buscema J., Carpenter S. E., Rosenshein N. B., Woodruff J. D. Epithelioid leiomyosarcoma of the uterus. Cancer. 1986 Mar 15;57(6):1192–1196. doi: 10.1002/1097-0142(19860315)57:6<1192::aid-cncr2820570621>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Buttram V. C., Jr, Reiter R. C. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981 Oct;36(4):433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- Castro H. F., Fechner R. E., Spjut H. J. Induced mesenchymal tumors of the rat genital tract. Arch Pathol. 1968 Nov;86(5):475–480. [PubMed] [Google Scholar]
- Cooper C. S., Stratton M. R. Soft tissue tumours: the genetic basis of development. Carcinogenesis. 1991 Feb;12(2):155–165. doi: 10.1093/carcin/12.2.155. [DOI] [PubMed] [Google Scholar]
- Devaney K., Tavassoli F. A. Immunohistochemistry as a diagnostic aid in the interpretation of unusual mesenchymal tumors of the uterus. Mod Pathol. 1991 Mar;4(2):225–231. [PubMed] [Google Scholar]
- Eker R., Mossige J., Johannessen J. V., Aars H. Hereditary renal adenomas and adenocarcinomas in rats. Diagn Histopathol. 1981 Jan-Mar;4(1):99–110. [PubMed] [Google Scholar]
- Evans H. L., Chawla S. P., Simpson C., Finn K. P. Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer. 1988 Nov 15;62(10):2239–2247. doi: 10.1002/1097-0142(19881115)62:10<2239::aid-cncr2820621028>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Everitt J. I., Goldsworthy T. L., Wolf D. C., Walker C. L. Hereditary renal cell carcinoma in the Eker rat: a rodent familial cancer syndrome. J Urol. 1992 Dec;148(6):1932–1936. doi: 10.1016/s0022-5347(17)37087-8. [DOI] [PubMed] [Google Scholar]
- Everitt J. I., Wolf D. C., Howe S. R., Goldsworthy T. L., Walker C. Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol. 1995 Jun;146(6):1556–1567. [PMC free article] [PubMed] [Google Scholar]
- Gopinath C., Gibson W. A. Mesovarian leiomyomas in the rat. Environ Health Perspect. 1987 Aug;73:107–113. doi: 10.1289/ehp.8773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino O., Klein-Szanto A. J., Freed J. J., Testa J. R., Brown D. Q., Vilensky M., Yeung R. S., Tartof K. D., Knudson A. G. Spontaneous and radiation-induced renal tumors in the Eker rat model of dominantly inherited cancer. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino O., Mitani H., Katsuyama H., Kubo Y. A novel cancer predisposition syndrome in the Eker rat model. Cancer Lett. 1994 Aug 15;83(1-2):117–121. doi: 10.1016/0304-3835(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kempson R. L., Hendrickson M. R. Pure mesenchymal neoplasms of the uterine corpus: selected problems. Semin Diagn Pathol. 1988 May;5(2):172–198. [PubMed] [Google Scholar]
- Kessel B., Liu J., Mortola J., Berga S., Yen S. S. Treatment of uterine fibroids with agonist analogs of gonadotropin-releasing hormone. Fertil Steril. 1988 Mar;49(3):538–541. [PubMed] [Google Scholar]
- Kurman R. J., Norris H. J. Mesenchymal tumors of the uterus. VI. Epithelioid smooth muscle tumors including leiomyoblastoma and clear-cell leiomyoma: a clinical and pathologic analysis of 26 cases. Cancer. 1976 Apr;37(4):1853–1865. doi: 10.1002/1097-0142(197604)37:4<1853::aid-cncr2820370433>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lium B., Moe L. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German shepherd dog: macroscopic and histopathologic changes. Vet Pathol. 1985 Sep;22(5):447–455. doi: 10.1177/030098588502200503. [DOI] [PubMed] [Google Scholar]
- Morton D., Mirsky M. A., Meier W. A., Thulin J. D. Vaginal stromal polyps in aging rats. Vet Pathol. 1994 Mar;31(2):287–289. doi: 10.1177/030098589403100228. [DOI] [PubMed] [Google Scholar]
- O'Connor D. M., Norris H. J. Mitotically active leiomyomas of the uterus. Hum Pathol. 1990 Feb;21(2):223–227. doi: 10.1016/0046-8177(90)90133-p. [DOI] [PubMed] [Google Scholar]
- Prayson R. A., Hart W. R. Mitotically active leiomyomas of the uterus. Am J Clin Pathol. 1992 Jan;97(1):14–20. doi: 10.1093/ajcp/97.1.14. [DOI] [PubMed] [Google Scholar]
- Rotmensch J., Bosnyak S., Montag A. Malignant transition of uterine leiomyomata. Int J Gynaecol Obstet. 1993 Jul;42(1):47–49. doi: 10.1016/0020-7292(93)90449-7. [DOI] [PubMed] [Google Scholar]
- Scully R. E. Smooth-muscle differentiation in genital tract disorders. Arch Pathol Lab Med. 1981 Oct;105(10):505–507. [PubMed] [Google Scholar]
- Shikora S. A., Niloff J. M., Bistrian B. R., Forse R. A., Blackburn G. L. Relationship between obesity and uterine leiomyomata. Nutrition. 1991 Jul-Aug;7(4):251–255. [PubMed] [Google Scholar]
- VAN VELSEN R. J., CROWLEY N. C. Centrosema mosaic: a plant virus disease transmitted by both aphids and plant bugs. Nature. 1961 Mar 11;189:858–858. doi: 10.1038/189858a0. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Jin F., Lee W. C., Testa J. R., Knudson A. G. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]










