Abstract
The mcl-1 gene encodes an approximately 37-kd protein that has significant homology with Bcl-2, an inhibitor of programmed cell death that is expressed in many types of long-lived cells. In this study we determined the in vivo patterns of Mcl-1 protein production in normal human tissues by immunohistochemical means, using specific polyclonal antisera, and made comparisons with Bcl-2. Like Bcl-2, Mcl-1 immunostaining was observed in epithelial cells in a variety of tissues, including prostate, breast, endometrium, epidermis, stomach, intestine, colon, and respiratory tract. However, often the expression of mcl-1 and bcl-2 in complex epithelia occurred in gradients with opposing directions, such that Bcl-2 immunostaining tended to be higher in the less differentiated cells lining the basement membrane, whereas Mcl-1 immunostaining was more intense in the differentiated cells located in the upper layers of these epithelia. The in vivo patterns of mcl-1 and bcl-2 expression were also strikingly different in several other tissues as well. Within the secondary follicles of lymph nodes and tonsils, for example, germinal center lymphocytes were Mcl-1 positive but mostly lacked Bcl-2; whereas mantle zone lymphocytes expressed bcl-2 but not mcl-1. Intense Mcl-1 immunoreactivity was also detected in several types of neuroendocrine cells, including the adrenal cortical cells that are Bcl-2 negative, sympathetic neurons that also contain Bcl-2, a subpopulation of cells in the pancreatic islets, Leydig cells of the testis, and granulosa lutein cells of the ovarian corpus luteum but not in thyroid epithelium, which is strongly Bcl-2 positive. Little or no Mcl-1 was detected in neurons in the brain and spinal cord, in contrast to Bcl-2, which is present in several types of central nervous system neurons. Conversely, strong Mcl-1 immunostaining was found in cardiac and skeletal muscle, which contain comparatively less Bcl-2. Additional types of cells that are Bcl-2-negative but that expressed mcl-1 include chondrocytes and hepatocytes. These findings demonstrate that mcl-1 expression is widespread in vivo and imply that the Mcl-1 and Bcl-2 proteins fulfill different roles in the overall physiology of cell death regulation.
Full text
PDF





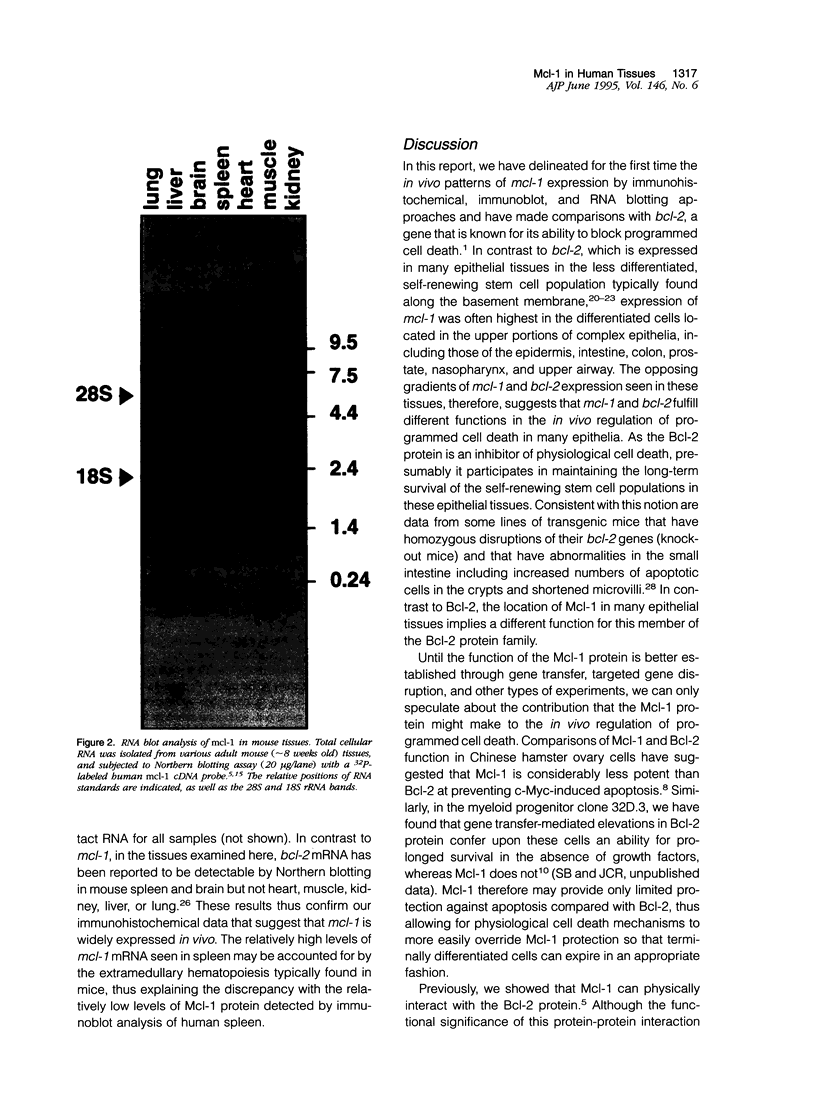


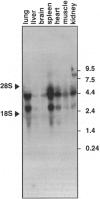
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borner C., Martinou I., Mattmann C., Irmler M., Schaerer E., Martinou J. C., Tschopp J. The protein bcl-2 alpha does not require membrane attachment, but two conserved domains to suppress apoptosis. J Cell Biol. 1994 Aug;126(4):1059–1068. doi: 10.1083/jcb.126.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner M. P., Culin C., Reed J. C., Furth E. E. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995 Jan;146(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kamada S., Shimono A., Shinto Y., Tsujimura T., Takahashi T., Noda T., Kitamura Y., Kondoh H., Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995 Jan 15;55(2):354–359. [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Bodrug S., Gascoyne R., Berean K., Krajewska M., Reed J. C. Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol. 1994 Sep;145(3):515–525. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Miyashita T., Wang H. G., Reed J. C. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994 Dec;145(6):1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Lin E. Y., Orlofsky A., Berger M. S., Prystowsky M. B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993 Aug 15;151(4):1979–1988. [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Branton P. E., Walton P. A., Oltvai Z. N., Korsmeyer S. J., Shore G. C. Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. J Biol Chem. 1994 Jun 17;269(24):16521–16524. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Meister L., Tanaka S., Cuddy M., Yum S., Geyer C., Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991 Dec 15;51(24):6529–6538. [PubMed] [Google Scholar]
- Reed J. C., Sabath D. E., Hoover R. G., Prystowsky M. B. Recombinant interleukin 2 regulates levels of c-myc mRNA in a cloned murine T lymphocyte. Mol Cell Biol. 1985 Dec;5(12):3361–3368. doi: 10.1128/mcb.5.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. E., Yang T., Qian L., Jenkinson J. D., Zhou P., Eastman A., Craig R. W. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994 Dec 15;54(24):6348–6352. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sato T., Hanada M., Bodrug S., Irie S., Iwama N., Boise L. H., Thompson C. B., Golemis E., Fong L., Wang H. G. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Saito K., Reed J. C. Structure-function analysis of the Bcl-2 oncoprotein. Addition of a heterologous transmembrane domain to portions of the Bcl-2 beta protein restores function as a regulator of cell survival. J Biol Chem. 1993 May 25;268(15):10920–10926. [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994 May 26;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- de Jong D., Prins F. A., Mason D. Y., Reed J. C., van Ommen G. B., Kluin P. M. Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994 Jan 1;54(1):256–260. [PubMed] [Google Scholar]