Abstract
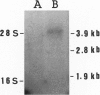
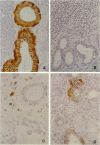
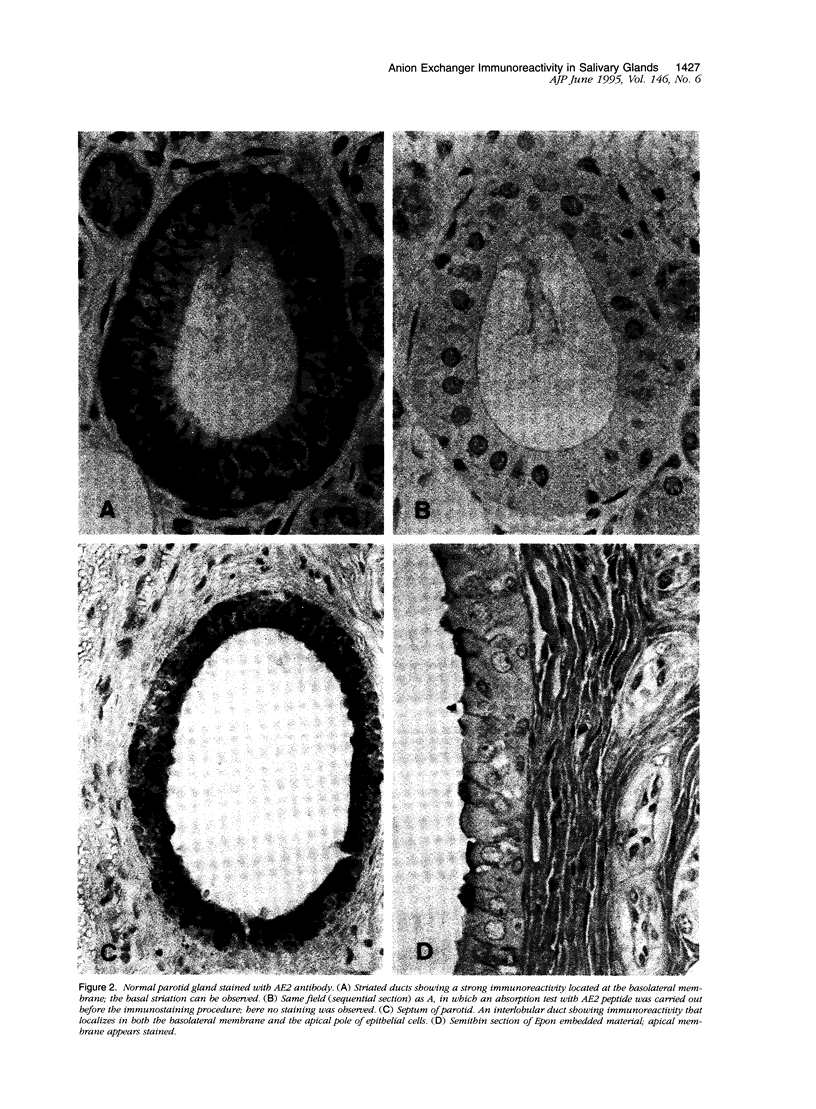
Salivary gland ducts play a relevant role in saliva secretion through transport processes. Na(+)-independent chloride-bicarbonate anion exchangers (AE) may be involved in these processes by generating ion fluxes into the salivary secretion. In Sjögren's syndrome, a disorder with gland dysfunction, there might be an impaired expression of AE proteins. Here we study AE immunoreactivities in human salivary glands, both in health and in Sjögren's syndrome. Immunohistochemistry was carried out on salivary glands from normal subjects and patients with Sjögren's syndrome, using two monoclonal antibodies against AE1 and AE2. Normal salivary glands showed AE2 immunoreactivity, which was restricted to the epithelium of the ducts, with no staining at the acini. A strong positivity was seen in the basolateral portion of the striated ducts, while interlobular duct cells showed a discrete positivity at their apical pole. In salivary glands from most of the patients with Sjögren's syndrome, AE2 immunoreactivity was absent in the ducts as well as in the acini. In both normal and diseased salivary glands, AE1 immunoreactivity was only located at the erythrocyte membrane. The recently reported AE0 was discarded because no AE0 message was found in salivary glands by reverse transcription polymerase chain reaction. In conclusion, AE2 immunoreactivity is observed in the ducts of normal salivary glands, particularly in the striated ducts. AE2 immunoreactivity is virtually absent in salivary glands from patients with Sjögren's syndrome, which may reflect either a loss of AE2 after inflammatory atrophy, or a primary defect occurring in the disease.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Alvaro D., Cho W. K., Mennone A., Boyer J. L. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993 Sep;92(3):1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Bruck R., Benedetti A., Strazzabosco M., Boyer J. L. Intracellular alkalinization stimulates bile flow and vesicular-mediated exocytosis in IPRL. Am J Physiol. 1993 Aug;265(2 Pt 1):G347–G353. doi: 10.1152/ajpgi.1993.265.2.G347. [DOI] [PubMed] [Google Scholar]
- Chisholm D. M., Mason D. K. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. 1968 Sep;21(5):656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chow A., Dobbins J. W., Aronson P. S., Igarashi P. cDNA cloning and localization of a band 3-related protein from ileum. Am J Physiol. 1992 Sep;263(3 Pt 1):G345–G352. doi: 10.1152/ajpgi.1992.263.3.G345. [DOI] [PubMed] [Google Scholar]
- Compton J. S., Nelson J., Wright R. D., Young J. A. A micropuncture investigation of electrolyte transport in the parotid glands of sodium-replete and sodium-depleted sheep. J Physiol. 1980 Dec;309:429–446. doi: 10.1113/jphysiol.1980.sp013518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gehrig H., Müller W., Appelhans H. Complete nucleotide sequence of band 3 related anion transport protein AE2 from human kidney. Biochim Biophys Acta. 1992 Apr 6;1130(3):326–328. doi: 10.1016/0167-4781(92)90446-7. [DOI] [PubMed] [Google Scholar]
- Havenga M. J., Bosman G. J., Appelhans H., De Grip W. J. Expression of the anion exchanger (AE) gene family in human brain. Identification of a new AE protein: AE0. Brain Res Mol Brain Res. 1994 Aug;25(1-2):97–104. doi: 10.1016/0169-328x(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. Bicarbonate secretion in rat ileum and its dependence on intraluminal chloride. Am J Physiol. 1967 Dec;213(6):1409–1413. doi: 10.1152/ajplegacy.1967.213.6.1409. [DOI] [PubMed] [Google Scholar]
- Knickelbein R. G., Aronson P. S., Dobbins J. W. Membrane distribution of sodium-hydrogen and chloride-bicarbonate exchangers in crypt and villus cell membranes from rabbit ileum. J Clin Invest. 1988 Dec;82(6):2158–2163. doi: 10.1172/JCI113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kopito R. R. Molecular biology of the anion exchanger gene family. Int Rev Cytol. 1990;123:177–199. doi: 10.1016/s0074-7696(08)60674-9. [DOI] [PubMed] [Google Scholar]
- Kudrycki K. E., Newman P. R., Shull G. E. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990 Jan 5;265(1):462–471. [PubMed] [Google Scholar]
- Lindsey A. E., Schneider K., Simmons D. M., Baron R., Lee B. S., Kopito R. R. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S. C., Kudrycki K. E., Shull G. E. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl-/HCO3- exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem. 1992 Apr 15;267(11):7927–7935. [PubMed] [Google Scholar]
- Lukacovic M. F., Feinstein M. B., Sha'afi R. I., Perrie S. Purification of stabilized band 3 protein of the human erythrocyte membrane and its reconstitution into liposomes. Biochemistry. 1981 May 26;20(11):3145–3151. doi: 10.1021/bi00514a025. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Ansó E., Castillo J. E., Díez J., Medina J. F., Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994 Jun;19(6):1400–1406. [PubMed] [Google Scholar]
- Mason M. J., Smith J. D., Garcia-Soto J. J., Grinstein S. Internal pH-sensitive site couples Cl-(-)HCO3- exchange to Na+-H+ antiport in lymphocytes. Am J Physiol. 1989 Feb;256(2 Pt 1):C428–C433. doi: 10.1152/ajpcell.1989.256.2.C428. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Medina J. F., Barrios C., Funk C. D., Larsson O., Haeggström J., Rådmark O. Human fibroblasts show expression of the leukotriene-A4-hydrolase gene, which is increased after simian-virus-40 transformation. Eur J Biochem. 1990 Jul 20;191(1):27–31. doi: 10.1111/j.1432-1033.1990.tb19089.x. [DOI] [PubMed] [Google Scholar]
- Melvin J. E., Turner R. J. Cl- fluxes related to fluid secretion by the rat parotid: involvement of Cl(-)-HCO3- exchange. Am J Physiol. 1992 Mar;262(3 Pt 1):G393–G398. doi: 10.1152/ajpgi.1992.262.3.G393. [DOI] [PubMed] [Google Scholar]
- Pirani D., Evans L. A., Cook D. I., Young J. A. Intracellular pH in the rat mandibular salivary gland: the role of Na-H and Cl-HCO3 antiports in secretion. Pflugers Arch. 1987 Feb;408(2):178–184. doi: 10.1007/BF00581349. [DOI] [PubMed] [Google Scholar]
- Prieto J., Qian C., García N., Díez J., Medina J. F. Abnormal expression of anion exchanger genes in primary biliary cirrhosis. Gastroenterology. 1993 Aug;105(2):572–578. doi: 10.1016/0016-5085(93)90735-u. [DOI] [PubMed] [Google Scholar]
- Shearn M. A. Sjögren's syndrome. Med Clin North Am. 1977 Mar;61(2):271–282. doi: 10.1016/s0025-7125(16)31332-3. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M., Mennone A., Boyer J. L. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991 May;87(5):1503–1512. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Tilley A., Sardet C., Pouyssegur J., Schwartz M. A., Brown D., Alper S. L. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol. 1994 Feb;266(2 Pt 1):C559–C568. doi: 10.1152/ajpcell.1994.266.2.C559. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. A., Machen T. E., Smolka A., Baron R., Kopito R. R. Identification of a 185-kDa band 3-related polypeptide in oxyntic cells. Am J Physiol. 1989 Sep;257(3 Pt 1):C537–C544. doi: 10.1152/ajpcell.1989.257.3.C537. [DOI] [PubMed] [Google Scholar]
- Turner R. J., George J. N., Baum B. J. Evidence for a Na+/K+/Cl- cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol. 1986;94(2):143–152. doi: 10.1007/BF01871194. [DOI] [PubMed] [Google Scholar]
- Turner R. J., George J. N. Cl(-)-HCO3- exchange is present with Na+-K+-Cl- cotransport in rabbit parotid acinar basolateral membranes. Am J Physiol. 1988 Mar;254(3 Pt 1):C391–C396. doi: 10.1152/ajpcell.1988.254.3.C391. [DOI] [PubMed] [Google Scholar]