Abstract
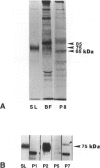
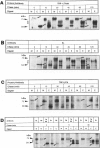
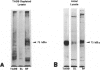
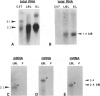
The Smyth line (SL) chicken is an animal model for the human acquired depigmentary disorder vitiligo. Affected birds from this line express a postnatal loss of melanocytes in feather and ocular tissues. This vitiligo-like depigmentation is considered to be a disorder with two interacting components: melanocyte dysfunctions and autoimmune reactions. Previously, SL chicks were shown to express high levels of circulating autoantibodies that bind to chicken melanocyte proteins with molecular masses between 65 and 80 kd. Three mammalian melanocyte proteins known to have isoforms in this molecular mass range are tyrosinase, tyrosinase-related protein (TRP)-1 and TRP-2. Of these, only tyrosinase is reported to be expressed in chicken melanocytes. The results presented in this study indicate that, of these three candidate proteins, TRP-1 is the primary antigen recognized by the SL autoantibodies. SL autoantibodies recognize a chicken melanocyte protein that is different from that of tyrosinase or the candidate chicken TRP-2. In addition, several types of experiments incriminate TRP-1 as the primary mammalian melanocyte antigen recognized by SL autoantibodies. We further verified that chicken melanocytes expressed messages for TRP-1 by finding positive signals on Northern blots of chicken melanocyte RNA probed with mammalian TRP-1 cDNA fragments. Therefore, we conclude from these results that the SL autoantibodies primarily recognize TRP-1 in mammalian melanocytes and suggest that chicken melanocytes express a homologue of TRP-1 (the human gp75 and the murine brown/b locus protein).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel Malek Z. A., Kreutzfeld K. L., Hadley M. E., Bregman M. D., Hruby V. J., Meyskens F. L., Jr Long-term and residual melanotropin-stimulated tyrosinase activity in S91 melanoma cells is density dependent. In Vitro Cell Dev Biol. 1986 Feb;22(2):75–81. doi: 10.1007/BF02623536. [DOI] [PubMed] [Google Scholar]
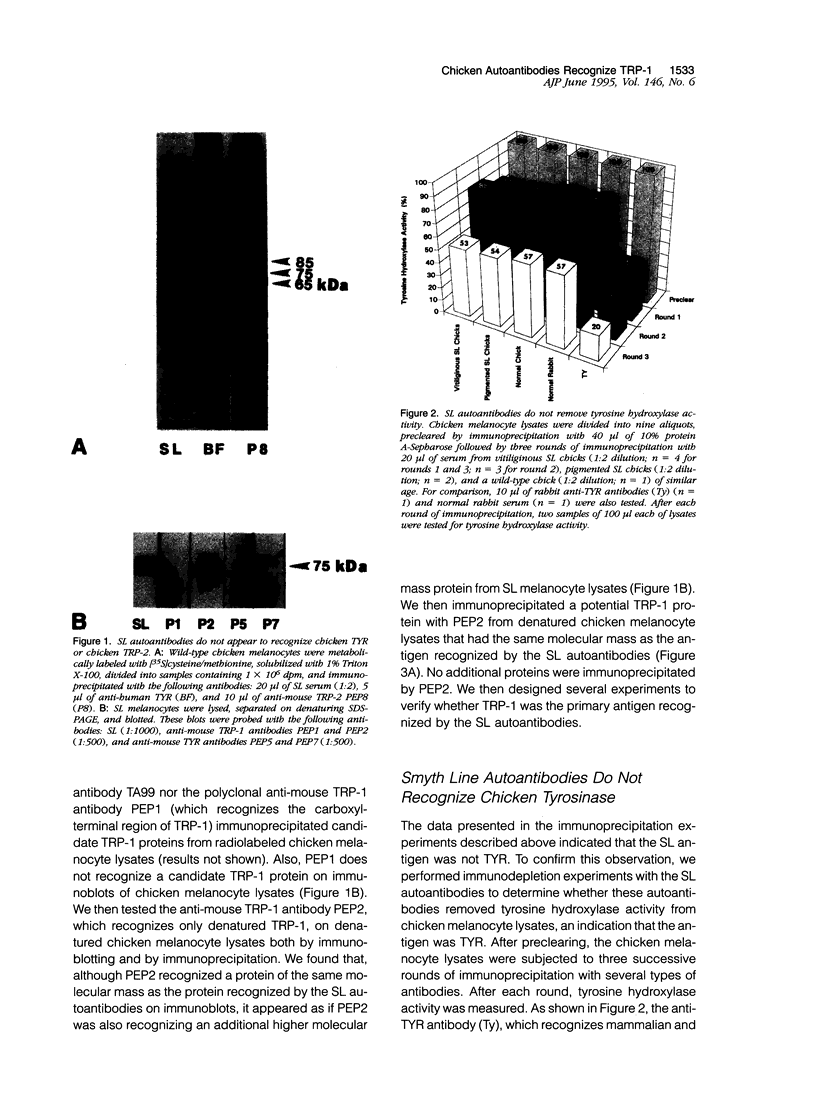
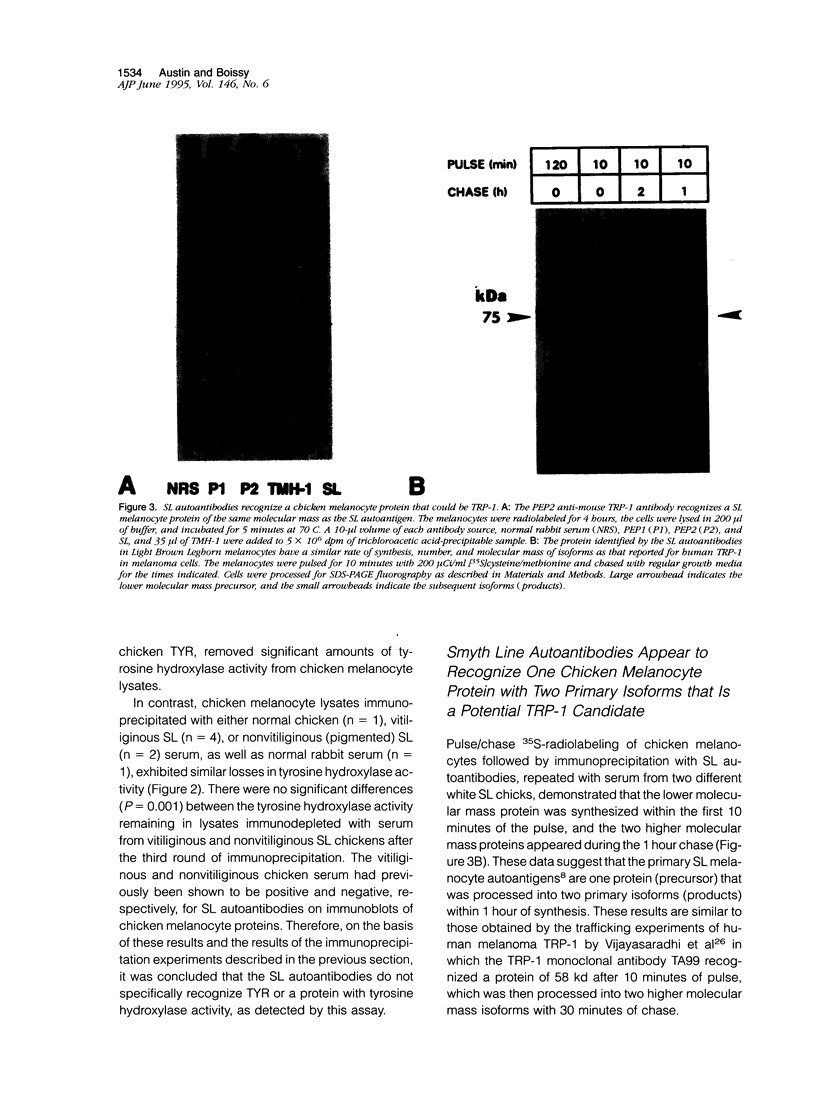
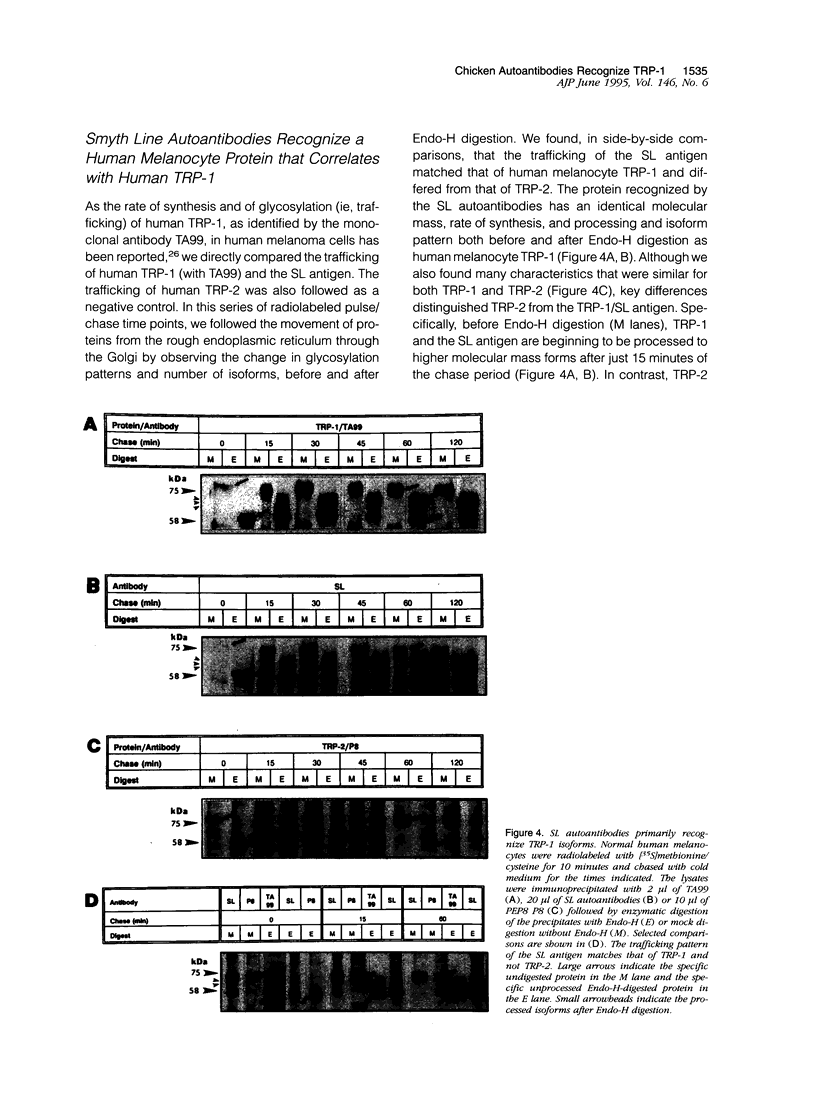
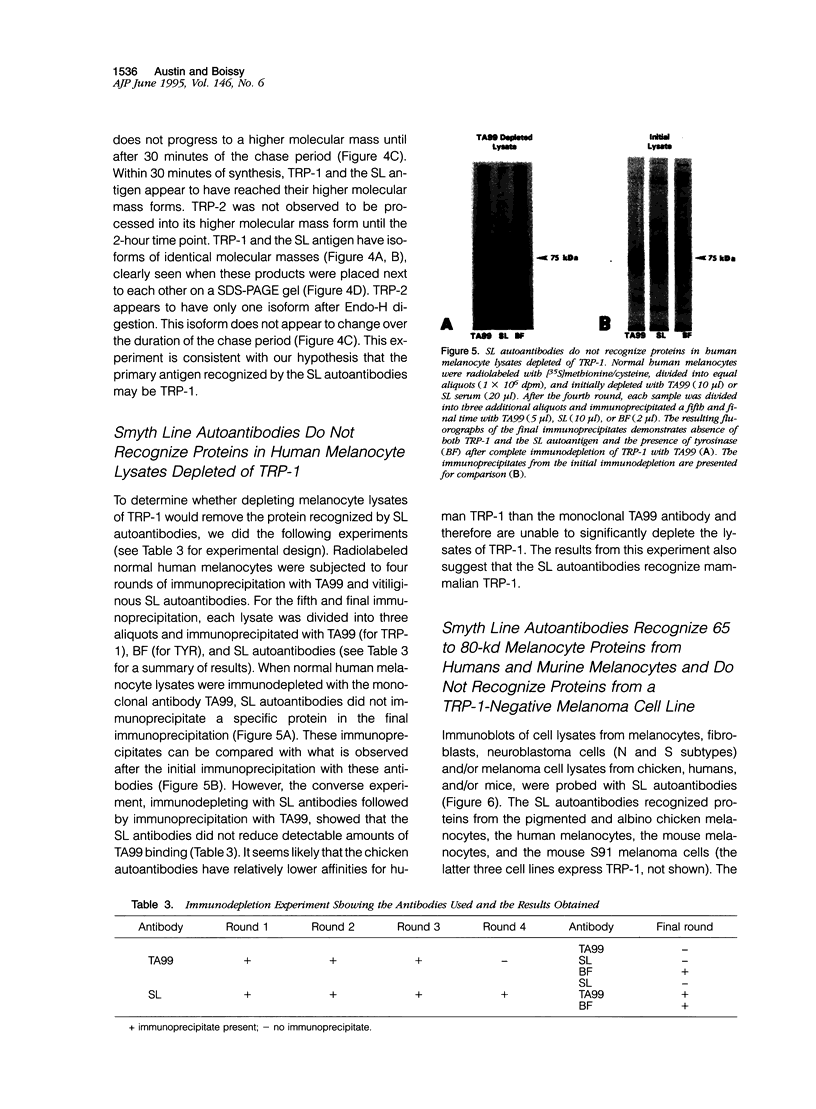
- Austin L. M., Boissy R. E., Jacobson B. S., Smyth J. R., Jr The detection of melanocyte autoantibodies in the Smyth chicken model for vitiligo. Clin Immunol Immunopathol. 1992 Aug;64(2):112–120. doi: 10.1016/0090-1229(92)90188-t. [DOI] [PubMed] [Google Scholar]
- Bennett D. C., Huszar D., Laipis P. J., Jaenisch R., Jackson I. J. Phenotypic rescue of mutant brown melanocytes by a retrovirus carrying a wild-type tyrosinase-related protein gene. Development. 1990 Oct;110(2):471–475. doi: 10.1242/dev.110.2.471. [DOI] [PubMed] [Google Scholar]
- Boissy R. E., Halaban R. Establishment of proliferative, pure cultures of pigmented chicken melanocytes from neural tubes. J Invest Dermatol. 1985 Feb;84(2):158–161. doi: 10.1111/1523-1747.ep12275408. [DOI] [PubMed] [Google Scholar]
- Boissy R. E., Lamont S. J., Smyth J. R., Jr Persistence of abnormal melanocytes in immunosuppressed chickens of the autoimmune "DAM" line. Cell Tissue Res. 1984;235(3):663–668. doi: 10.1007/BF00226966. [DOI] [PubMed] [Google Scholar]
- Boissy R. E., Lamoreux M. L. Animal models of an acquired pigmentary disorder--vitiligo. Prog Clin Biol Res. 1988;256:207–218. [PubMed] [Google Scholar]
- Bouchard B., Fuller B. B., Vijayasaradhi S., Houghton A. N. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J Exp Med. 1989 Jun 1;169(6):2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. L., 3rd, Pardue S. L., Smyth J. R., Jr Effect of corticosterone on the incidence of amelanosis in Smyth delayed amelanotic line chickens. Poult Sci. 1987 Feb;66(2):363–367. doi: 10.3382/ps.0660363. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cui J., Harning R., Henn M., Bystryn J. C. Identification of pigment cell antigens defined by vitiligo antibodies. J Invest Dermatol. 1992 Feb;98(2):162–165. doi: 10.1111/1523-1747.ep12555773. [DOI] [PubMed] [Google Scholar]
- Foley J., Cohn S. L., Salwen H. R., Chagnovich D., Cowan J., Mason K. L., Parysek L. M. Differential expression of N-myc in phenotypically distinct subclones of a human neuroblastoma cell line. Cancer Res. 1991 Dec 1;51(23 Pt 1):6338–6345. [PubMed] [Google Scholar]
- Fuller B. B., Lunsford J. B., Iman D. S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987 Mar 25;262(9):4024–4033. [PubMed] [Google Scholar]
- Giacomini P., Fraioli R., Cuomo M., Natali P. G. Membrane compartmentalization of melanosomal gp75. J Invest Dermatol. 1992 Mar;98(3):340–342. doi: 10.1111/1523-1747.ep12499801. [DOI] [PubMed] [Google Scholar]
- Guerra L., Mordoh J., Slavutsky I., Larripa I., Medrano E. E. Characterization of IIB-MEL-J: a new and highly heterogenous human melanoma cell line. Pigment Cell Res. 1989 Nov-Dec;2(6):504–509. doi: 10.1111/j.1600-0749.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Halaban R., Moellmann G. Recent advances in the molecular biology of pigmentation: mouse models. Pigment Cell Res. 1992;Suppl 2:67–78. doi: 10.1111/j.1600-0749.1990.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Halaban R., Pomerantz S. H., Marshall S., Lambert D. T., Lerner A. B. Regulation of tyrosinase in human melanocytes grown in culture. J Cell Biol. 1983 Aug;97(2):480–488. doi: 10.1083/jcb.97.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harning R., Cui J., Bystryn J. C. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991 Dec;97(6):1078–1080. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K., Urabe K., Kameyama K., Montague P. M., Jackson I. J. Functional properties of cloned melanogenic proteins. Pigment Cell Res. 1992 Nov;5(5 Pt 2):264–270. doi: 10.1111/j.1600-0749.1992.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Cervantes C., Solano F., Kobayashi T., Urabe K., Hearing V. J., Lozano J. A., García-Borrón J. C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J Biol Chem. 1994 Jul 8;269(27):17993–18000. [PubMed] [Google Scholar]
- Jiménez M., Maloy W. L., Hearing V. J. Specific identification of an authentic clone for mammalian tyrosinase. J Biol Chem. 1989 Feb 25;264(6):3397–3403. [PubMed] [Google Scholar]
- Jiménez M., Tsukamoto K., Hearing V. J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991 Jan 15;266(2):1147–1156. [PubMed] [Google Scholar]
- Kameyama K., Takemura T., Hamada Y., Sakai C., Kondoh S., Nishiyama S., Urabe K., Hearing V. J. Pigment production in murine melanoma cells is regulated by tyrosinase, tyrosinase-related protein 1 (TRP1), DOPAchrome tautomerase (TRP2), and a melanogenic inhibitor. J Invest Dermatol. 1993 Feb;100(2):126–131. doi: 10.1111/1523-1747.ep12462778. [DOI] [PubMed] [Google Scholar]
- Lamont S. J., Smyth J. R., Jr Effect of bursectomy on development of a spontaneous postnatal amelanosis. Clin Immunol Immunopathol. 1981 Dec;21(3):407–411. doi: 10.1016/0090-1229(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Thomson T. M., Old L. J., Lloyd K. O. A pigmentation-associated, differentiation antigen of human melanoma defined by a precipitating antibody in human serum. Int J Cancer. 1983 Dec 15;32(6):717–721. doi: 10.1002/ijc.2910320610. [DOI] [PubMed] [Google Scholar]
- Medrano E. E., Nordlund J. J. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. J Invest Dermatol. 1990 Oct;95(4):441–445. [PubMed] [Google Scholar]
- Naughton G. K., Eisinger M., Bystryn J. C. Antibodies to normal human melanocytes in vitiligo. J Exp Med. 1983 Jul 1;158(1):246–251. doi: 10.1084/jem.158.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Kissinger R. M., Naughton G. M., Bystryn J. C. Evidence for immunologic mechanisms in human vitiligo: patients' sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol. 1988 Jun;90(6):783–789. doi: 10.1111/1523-1747.ep12461505. [DOI] [PubMed] [Google Scholar]
- Oetting W., Langner K., Brumbaugh J. A. Detection of melanogenic proteins in cultured chick-embryo melanocytes. Differentiation. 1985;30(1):40–46. doi: 10.1111/j.1432-0436.1985.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Ortonne J. P., Bose S. K. Vitiligo: where do we stand? Pigment Cell Res. 1993 Mar;6(2):61–72. doi: 10.1111/j.1600-0749.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Pardue S. L., Fite K. V., Bengston L., Lamont S. J., Boyle M. L., 3rd, Smyth J. R., Jr Enhanced integumental and ocular amelanosis following the termination of cyclosporine administration. J Invest Dermatol. 1987 Jun;88(6):758–761. doi: 10.1111/1523-1747.ep12470453. [DOI] [PubMed] [Google Scholar]
- Searle E. A., Austin L. M., Boissy Y. L., Zhao H., Nordlund J. J., Boissy R. E. Smyth chicken melanocyte autoantibodies: cross-species recognition, in vivo binding, and plasma membrane reactivity of the antiserum. Pigment Cell Res. 1993 Jun;6(3):145–157. doi: 10.1111/j.1600-0749.1993.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. H., Connor E., Li Y., Zorovich B., Balducci P., Maclaren N. The role of tyrosinase in autoimmune vitiligo. Lancet. 1994 Oct 15;344(8929):1049–1052. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- Swope V. B., Abdel-Malek Z., Kassem L. M., Nordlund J. J. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991 Feb;96(2):180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- Tamura A., Halaban R., Moellmann G., Cowan J. M., Lerner M. R., Lerner A. B. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987 Jul;23(7):519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Shibahara S., Takeda A., Okinaga S., Matsunaga J., Tagami H. The monoclonal antibodies TMH-1 and TMH-2 specifically bind to a protein encoded at the murine b-locus, not to the authentic tyrosinase encoded at the c-locus. J Invest Dermatol. 1991 Apr;96(4):500–504. doi: 10.1111/1523-1747.ep12470204. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasaradhi S., Bouchard B., Houghton A. N. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med. 1990 Apr 1;171(4):1375–1380. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasaradhi S., Doskoch P. M., Houghton A. N. Biosynthesis and intracellular movement of the melanosomal membrane glycoprotein gp75, the human b (brown) locus product. Exp Cell Res. 1991 Oct;196(2):233–240. doi: 10.1016/0014-4827(91)90256-t. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Brumbaugh J. A. Purification and isoelectric heterogeneity of chicken tyrosinase. Biochim Biophys Acta. 1984 Aug 21;800(3):282–290. doi: 10.1016/0304-4165(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Zdarsky E., Favor J., Jackson I. J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics. 1990 Oct;126(2):443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhao Y., Nordlund J. J., Boissy R. E. Human TRP-1 has tyrosine hydroxylase but no dopa oxidase activity. Pigment Cell Res. 1994 Jun;7(3):131–140. doi: 10.1111/j.1600-0749.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]