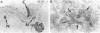Abstract
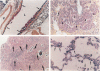
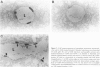
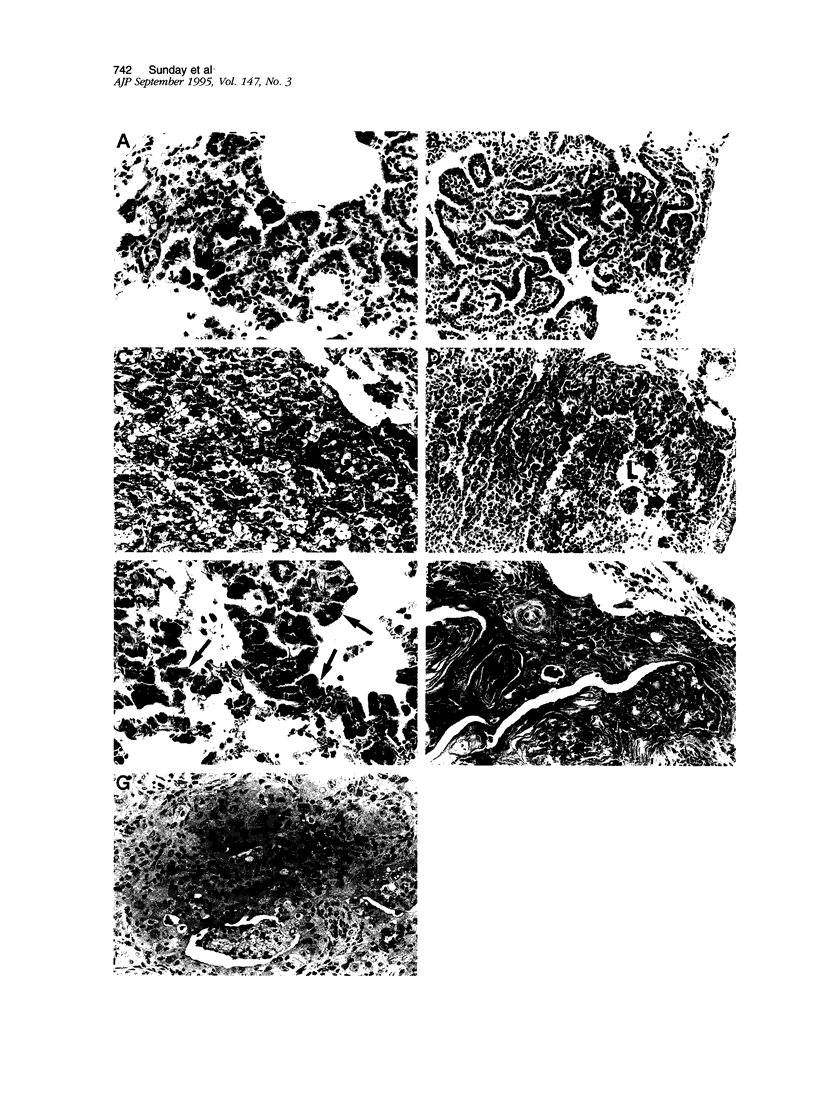
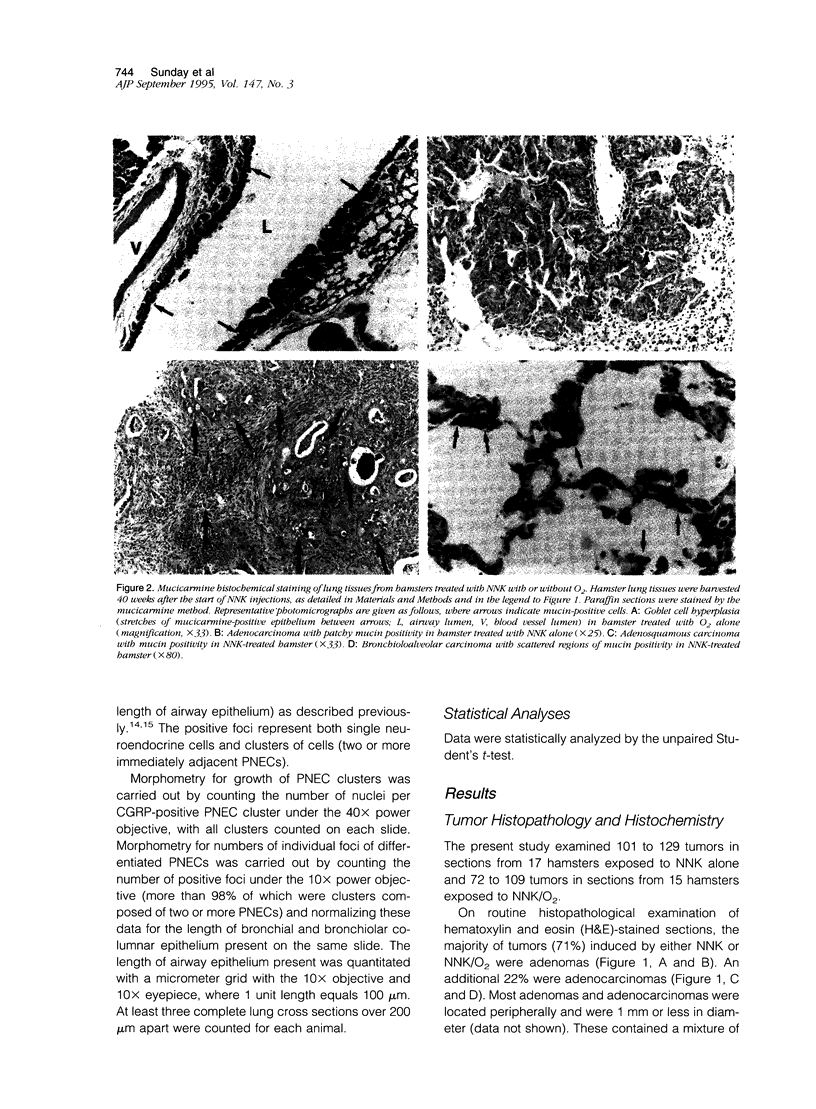
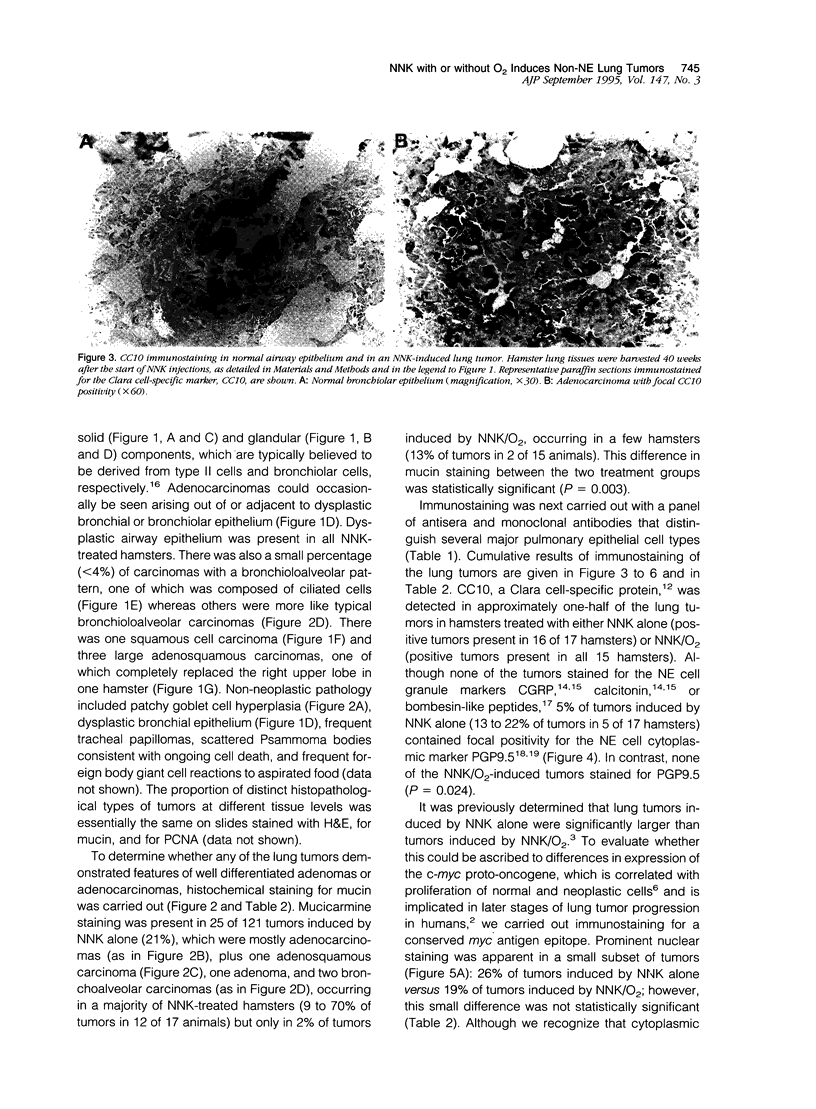
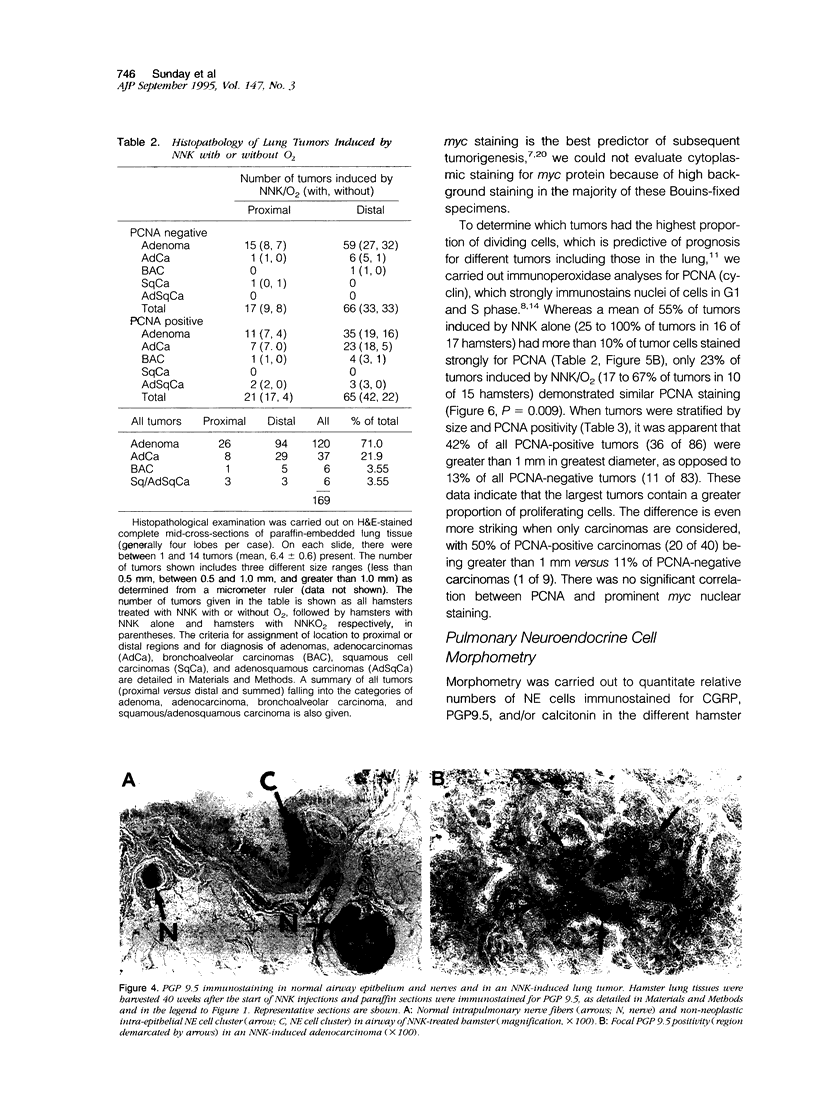
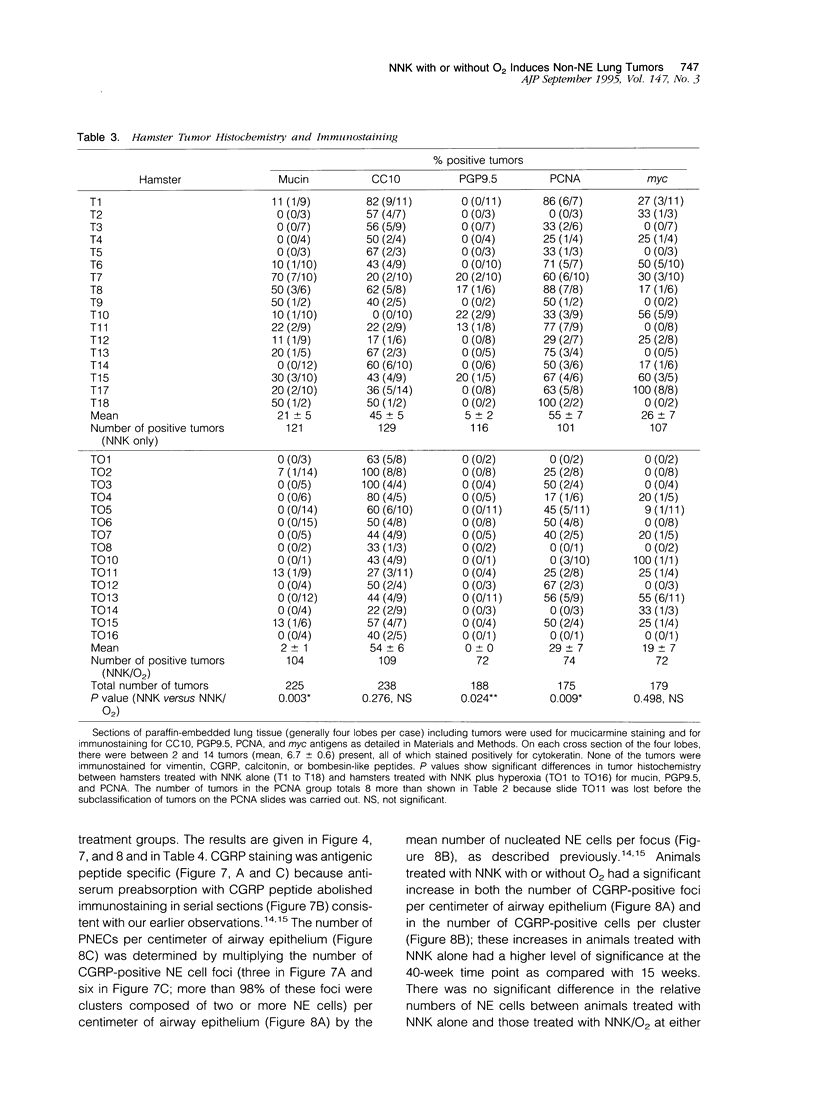
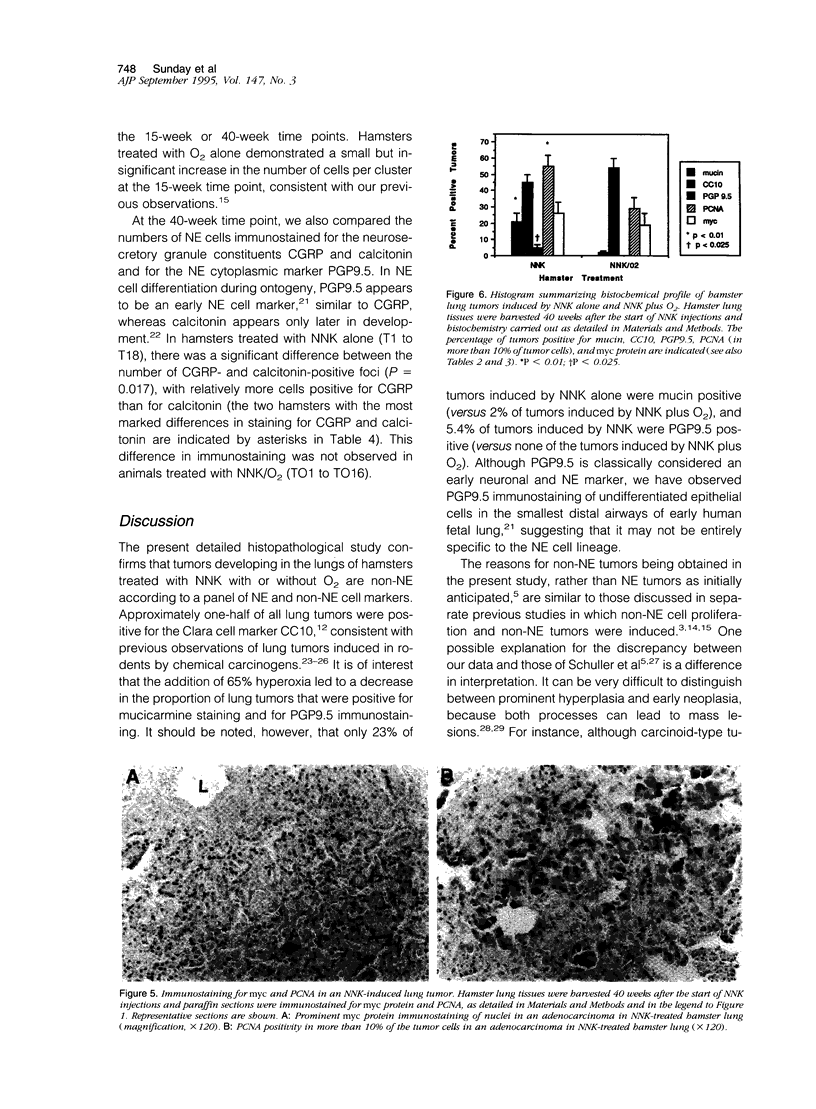
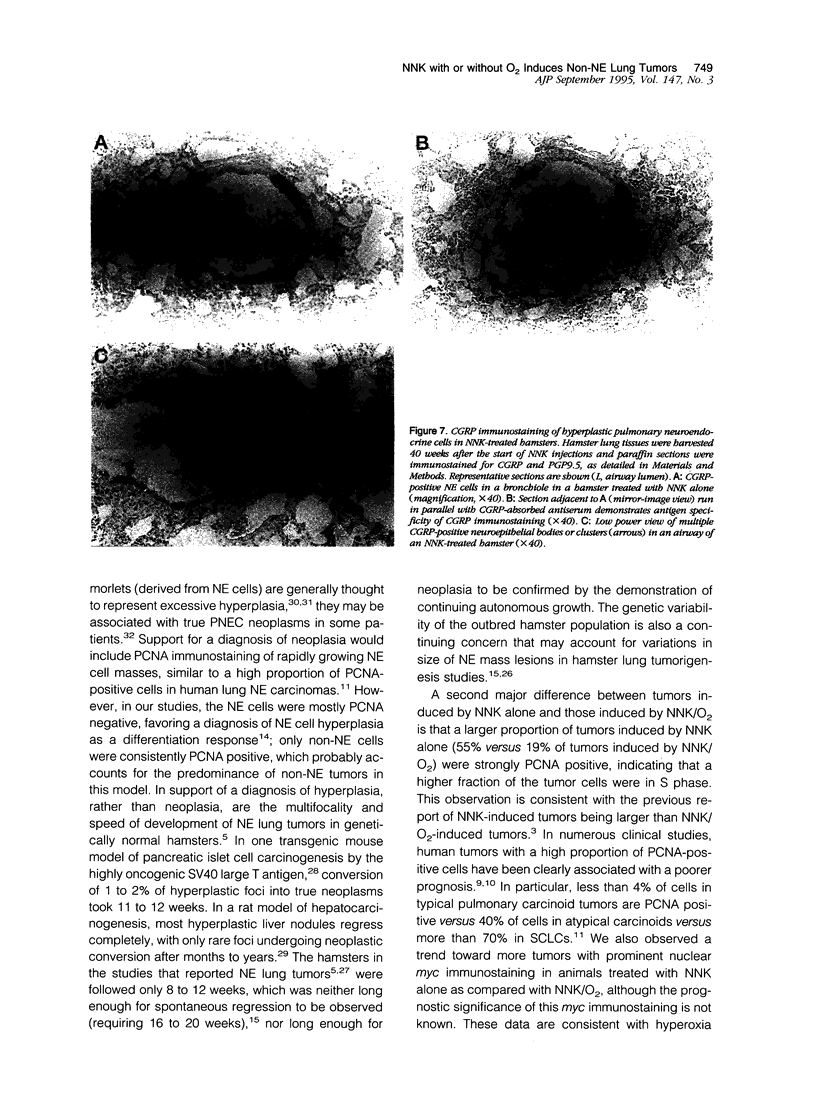
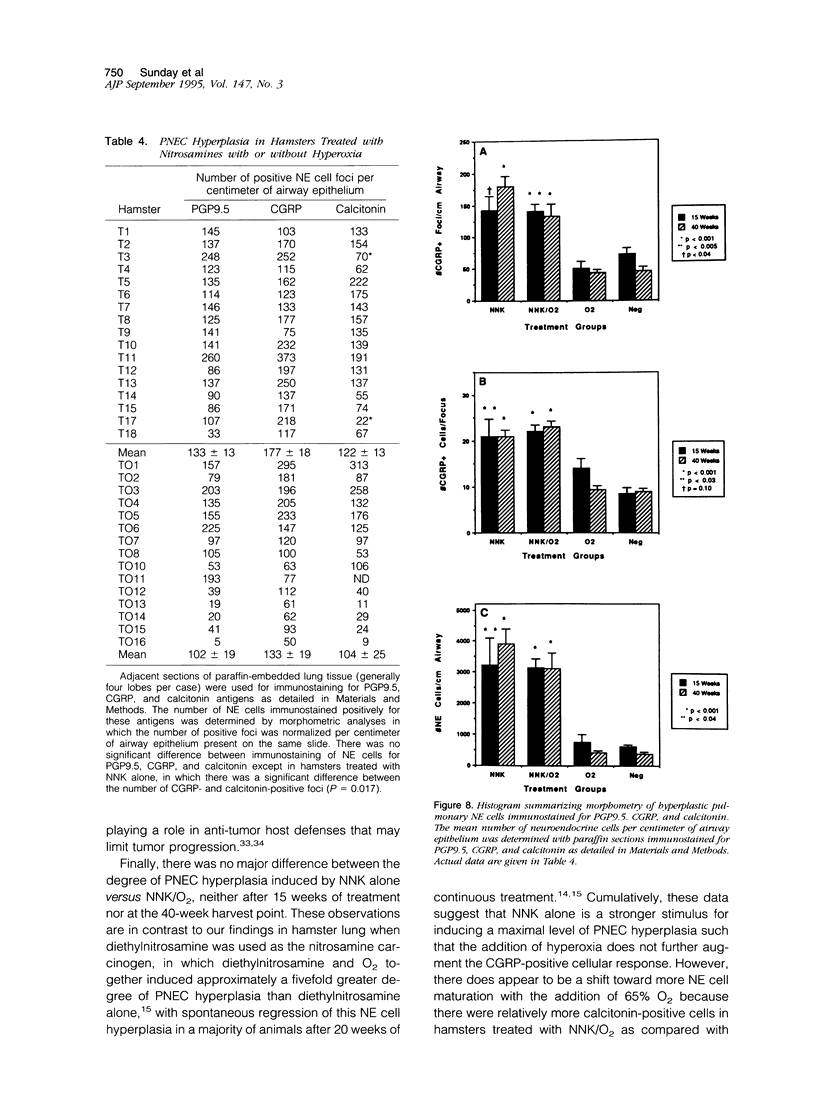
Lung tumors induced by 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) with or without hyperoxia have frequent K-ras mutations but only rare p53 mutations, suggesting that this may be a model for non-small cell lung cancers. The goals of the present study were (1) to characterize the histopathology of lung tumors induced in hamsters by NNK with or without O2 and (2) as a corollary, to quantitate the pulmonary neuroendocrine cell hyperplasia in the different treatment groups early and late in the treatment period. Lung tumors induced by NNK with or without O2 were 71% adenomas, 22% adenocarcinomas, approximately 4% bronchoalveolar carcinomas, and approximately 4% squamous/adenosquamous carcinomas. One-half of all tumors were positive for the Clara cell antigen CC10 and 21% of NNK-induced tumors were mucin positive, compared with 2% of NNK/O2-induced tumors (P = 0.003). Immunostaining for PGP9.5 was positive in 5% of tumors induced by NNK alone, but in none of NNK/O2-induced tumors (P = 0.024). Abundant proliferating cell nuclear antigen occurred in 55% of NNK-induced tumors, compared with 19% of NNK/O2-induced tumors (P = 0.009). These data indicate that NNK with or without O2 induces non-neuroendocrine lung tumors. Hyperoxia appears to inhibit cell proliferation and suppress mucinous and partial neuroendocrine differentiation in some of these tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo S. M., Miller Y. E., Waldron J. A., Jr, Bogin R. M., Sunday M. E., Staton G. W., Jr, Beam W. R., King T. E., Jr Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992 Oct 29;327(18):1285–1288. doi: 10.1056/NEJM199210293271806. [DOI] [PubMed] [Google Scholar]
- Barbareschi M., Girlando S., Mauri F. A., Arrigoni G., Laurino L., Dalla Palma P., Doglioni C. Tumour suppressor gene products, proliferation, and differentiation markers in lung neuroendocrine neoplasms. J Pathol. 1992 Apr;166(4):343–350. doi: 10.1002/path.1711660405. [DOI] [PubMed] [Google Scholar]
- Becker K. L., O'Neil W. J., Snider R. H., Jr, Nylen E. S., Moore C. F., Jeng J., Silva O. L., Lewis M. S., Jordan M. H. Hypercalcitonemia in inhalation burn injury: a response of the pulmonary neuroendocrine cell? Anat Rec. 1993 May;236(1):136-8, 172-3; discussion 138-43. doi: 10.1002/ar.1092360118. [DOI] [PubMed] [Google Scholar]
- Birrer M. J., Brown P. H. Application of molecular genetics to the early diagnosis and screening of lung cancer. Cancer Res. 1992 May 1;52(9 Suppl):2658s–2664s. [PubMed] [Google Scholar]
- Bohn W., Wiegers W., Beuttenmüller M., Traub P. Species-specific recognition patterns of monoclonal antibodies directed against vimentin. Exp Cell Res. 1992 Jul;201(1):1–7. doi: 10.1016/0014-4827(92)90341-5. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Dervan P. A., Magee H. M., Buckley C., Carney D. N. Proliferating cell nuclear antigen counts in formalin-fixed paraffin-embedded tissue correlate with Ki-67 in fresh tissue. Am J Clin Pathol. 1992 May;97(5 Suppl 1):S21–S28. [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Fujii K., Odashima S., Okada M. Induction of tumours by administration of N-dibutylnitrosamine and derivatives to infant mice. Br J Cancer. 1977 May;35(5):610–614. doi: 10.1038/bjc.1977.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Linnoila R. I., Memoli V. A., Warren W. H. Neuroendocrine components of the bronchopulmonary tract: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1983 Nov;49(5):519–537. [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Georgieff M. K. Pulmonary neuroendocrine cells. Their secretory products and their potential roles in health and chronic lung disease in infancy. Am Rev Respir Dis. 1989 Dec;140(6):1807–1812. doi: 10.1164/ajrccm/140.6.1807. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Wobken J. D., Landrum B. G. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis. 1988 Jan;137(1):123–131. doi: 10.1164/ajrccm/137.1.123. [DOI] [PubMed] [Google Scholar]
- Kato G. J., Dang C. V. Function of the c-Myc oncoprotein. FASEB J. 1992 Sep;6(12):3065–3072. doi: 10.1096/fasebj.6.12.1521738. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R. C., Margaretten N., Griesemer R. A., Witschi H. P. Modification of lung tumor growth by hyperoxia. Carcinogenesis. 1986 Sep;7(9):1581–1586. doi: 10.1093/carcin/7.9.1581. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R. C., Tryka A. F., Witschi H. P. Inhibition of mouse lung tumor development by hyperoxia. Cancer Res. 1986 Apr;46(4 Pt 2):1994–2000. [PubMed] [Google Scholar]
- Linnoila R. I., Jensen S. M., Steinberg S. M., Mulshine J. L., Eggleston J. C., Gazdar A. F. Peripheral airway cell marker expression in non-small cell lung carcinoma. Association with distinct clinicopathologic features. Am J Clin Pathol. 1992 Feb;97(2):233–243. doi: 10.1093/ajcp/97.2.233. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K., Eskelinen M. J. Cell proliferation of transitional cell bladder tumours determined by PCNA/cyclin immunostaining and its prognostic value. Br J Cancer. 1992 Jul;66(1):171–176. doi: 10.1038/bjc.1992.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. C., Rao K. V., Detrisac C. J., Kelloff G. J. Hamster lung cancer model of carcinogenesis and chemoprevention. Adv Exp Med Biol. 1992;320:55–61. doi: 10.1007/978-1-4615-3468-6_8. [DOI] [PubMed] [Google Scholar]
- Nettesheim P., Marchok A. Neoplastic development in airway epithelium. Adv Cancer Res. 1983;39:1–70. doi: 10.1016/s0065-230x(08)61032-5. [DOI] [PubMed] [Google Scholar]
- Oreffo V. I., Lin H. W., Gumerlock P. H., Kraegel S. A., Witschi H. Mutational analysis of a dominant oncogene (c-Ki-ras-2) and a tumor suppressor gene (p53) in hamster lung tumorigenesis. Mol Carcinog. 1992;6(3):199–202. doi: 10.1002/mc.2940060305. [DOI] [PubMed] [Google Scholar]
- Oreffo V. I., Lin H. W., Padmanabhan R., Witschi H. K-ras and p53 point mutations in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced hamster lung tumors. Carcinogenesis. 1993 Mar;14(3):451–455. doi: 10.1093/carcin/14.3.451. [DOI] [PubMed] [Google Scholar]
- Roncalli M., Springall D. R., Varndell I. M., Gaitonde V. V., Hamid Q., Ibrahim N. B., Grimelius L., Wilander E., Polak J. M., Coggi G. Oncoprotein immunoreactivity in human endocrine tumours. J Pathol. 1991 Feb;163(2):117–127. doi: 10.1002/path.1711630207. [DOI] [PubMed] [Google Scholar]
- Royds J. A., Sharrard R. M., Wagner B., Polacarz S. V. Cellular localisation of c-myc product in human colorectal epithelial neoplasia. J Pathol. 1992 Mar;166(3):225–233. doi: 10.1002/path.1711660304. [DOI] [PubMed] [Google Scholar]
- Schuller H. M., Becker K. L., Witschi H. P. An animal model for neuroendocrine lung cancer. Carcinogenesis. 1988 Feb;9(2):293–296. doi: 10.1093/carcin/9.2.293. [DOI] [PubMed] [Google Scholar]
- Schuller H. M., Correa E., Orloff M., Reznik G. K. Successful chemotherapy of experimental neuroendocrine lung tumors in hamsters with an antagonist of Ca2+/calmodulin. Cancer Res. 1990 Mar 1;50(5):1645–1649. [PubMed] [Google Scholar]
- Singh G., Singh J., Katyal S. L., Brown W. E., Kramps J. A., Paradis I. L., Dauber J. H., Macpherson T. A., Squeglia N. Identification, cellular localization, isolation, and characterization of human Clara cell-specific 10 KD protein. J Histochem Cytochem. 1988 Jan;36(1):73–80. doi: 10.1177/36.1.3275712. [DOI] [PubMed] [Google Scholar]
- Slebos R. J., Kibbelaar R. E., Dalesio O., Kooistra A., Stam J., Meijer C. J., Wagenaar S. S., Vanderschueren R. G., van Zandwijk N., Mooi W. J. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990 Aug 30;323(9):561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- Springall D. R., Ibrahim N. B., Rode J., Sharpe M. S., Bloom S. R., Polak J. M. Endocrine differentiation of extra-pulmonary small cell carcinoma demonstrated by immunohistochemistry using antibodies to PGP 9.5, neuron-specific enolase and the C-flanking peptide of human pro-bombesin. J Pathol. 1986 Nov;150(3):151–162. doi: 10.1002/path.1711500302. [DOI] [PubMed] [Google Scholar]
- Stahlman M. T., Kasselberg A. G., Orth D. N., Gray M. E. Ontogeny of neuroendocrine cells in human fetal lung. II. An immunohistochemical study. Lab Invest. 1985 Jan;52(1):52–60. [PubMed] [Google Scholar]
- Sunday M. E., Choi N., Spindel E. R., Chin W. W., Mark E. J. Gastrin-releasing peptide gene expression in small cell and large cell undifferentiated lung carcinomas. Hum Pathol. 1991 Oct;22(10):1030–1039. doi: 10.1016/0046-8177(91)90011-d. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Hua J., Dai H. B., Nusrat A., Torday J. S. Bombesin increases fetal lung growth and maturation in utero and in organ culture. Am J Respir Cell Mol Biol. 1990 Sep;3(3):199–205. doi: 10.1165/ajrcmb/3.3.199. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Willett C. G. Induction and spontaneous regression of intense pulmonary neuroendocrine cell differentiation in a model of preneoplastic lung injury. Cancer Res. 1992 May 1;52(9 Suppl):2677s–2686s. [PubMed] [Google Scholar]
- Sunday M. E., Willett C. G., Patidar K., Graham S. A. Modulation of oncogene and tumor suppressor gene expression in a hamster model of chronic lung injury with varying degrees of pulmonary neuroendocrine cell hyperplasia. Lab Invest. 1994 Jun;70(6):875–888. [PubMed] [Google Scholar]
- Thompson R. J., Doran J. F., Jackson P., Dhillon A. P., Rode J. PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983 Nov 14;278(1-2):224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Rao K. V., Mihailovich N. Neoplastic response of mouse tissues during perinatal age periods and its significance in chemical carcinogenesis. Natl Cancer Inst Monogr. 1979 May;(51):239–250. [PubMed] [Google Scholar]
- Watanabe H., Kobayashi H., Honma K., Ohnishi Y., Iwafuchi M. Diffuse panbronchiolitis with multiple tumorlets. A quantitative study of the Kultschitzky cells and the clusters. Acta Pathol Jpn. 1985 Sep;35(5):1221–1231. [PubMed] [Google Scholar]
- Willett C. G., Warland G., Cheek R., Coen J., Efird J., Shellito P. C., Compton C. C. Proliferating cell nuclear antigen and mitotic activity in rectal cancer: predictor of response to preoperative irradiation. J Clin Oncol. 1994 Apr;12(4):679–682. doi: 10.1200/JCO.1994.12.4.679. [DOI] [PubMed] [Google Scholar]