Abstract
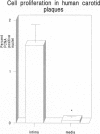
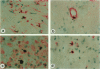
Cell proliferation, an important mechanism of atherosclerotic plaque growth, occurs among smooth muscle, inflammatory cell, and other cell types. We have identified different topographical patterns of cell proliferation in human carotid plaques, based on cell type. Cell proliferation was determined with an antibody to the proliferating cell nuclear antigen (PCNA), combined with cell type-specific antibodies. Despite low levels of overall proliferative activity, the intima displayed more proliferative activity than the underlying media (1.61 +/- 0.35% in intima versus 0.05 +/- 0.03% in media; P < 0.01). The preponderant proliferative cell type in the intima was the monocyte/macrophage (46.0% of PCNA-positive cells), with a minority being smooth muscle alpha-actin-positive (9.7%), microvascular endothelial (14.3%), and T cells (13.1%). Smooth muscle cells were the dominant proliferating cell type in the media (44.4% of PCNA-positive cells versus 20% endothelial cells, 13.0% monocyte/macrophages, and 14.3% T cells). Within the plaque, foam-cell-rich regions mostly displayed proliferation among macrophages (66.5%), whereas in vascularized fields PCNA positivity was almost equally shared by endothelial cells (23.8%), monocyte/macrophages (26.3%), smooth muscle alpha-actin-positive cells (14.0%), and to a lesser extent, T cells (8.2%). Logistic and linear regression analyses also demonstrated that location in foam-cell-rich regions was a significant predictor of proliferation only among monocyte/macrophages, whereas location in vascularized regions was a good predictor of PCNA positivity among both inflammatory and noninflammatory cells. These different patterns of cell type proliferation suggest possibly different distributions of putative responsible growth regulatory factors in human atherosclerosis.
Full text
PDF


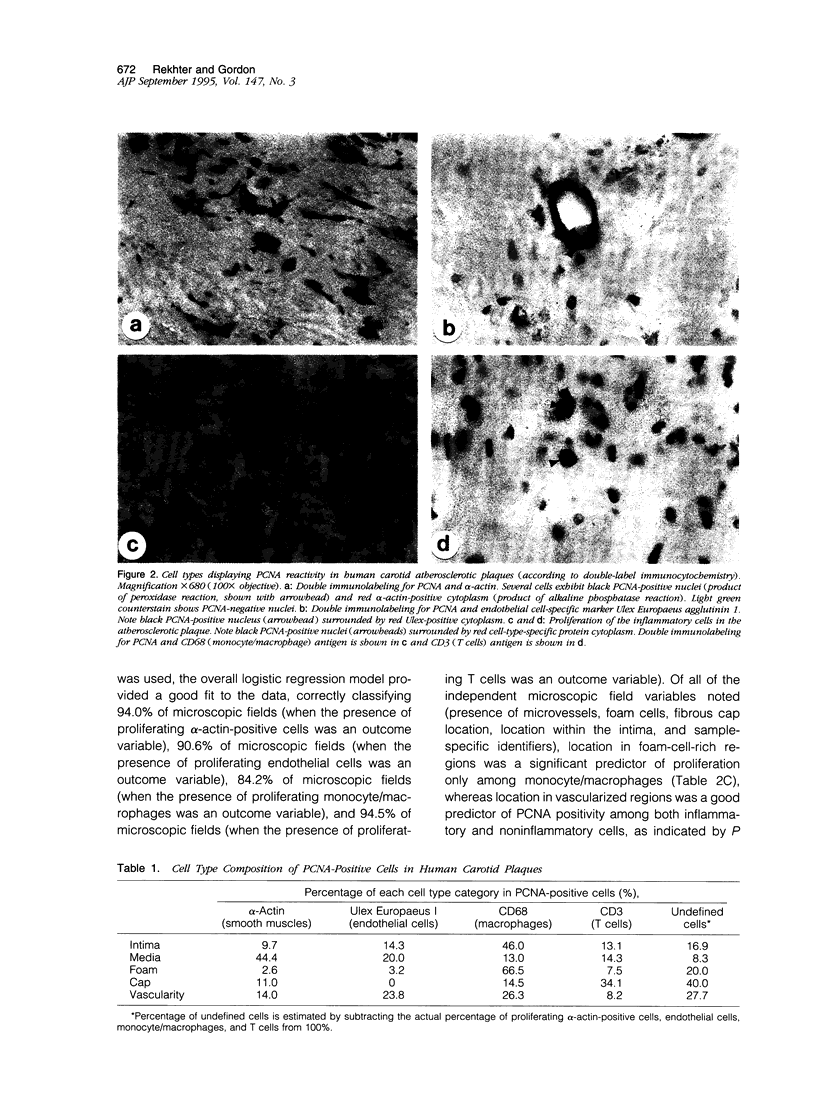
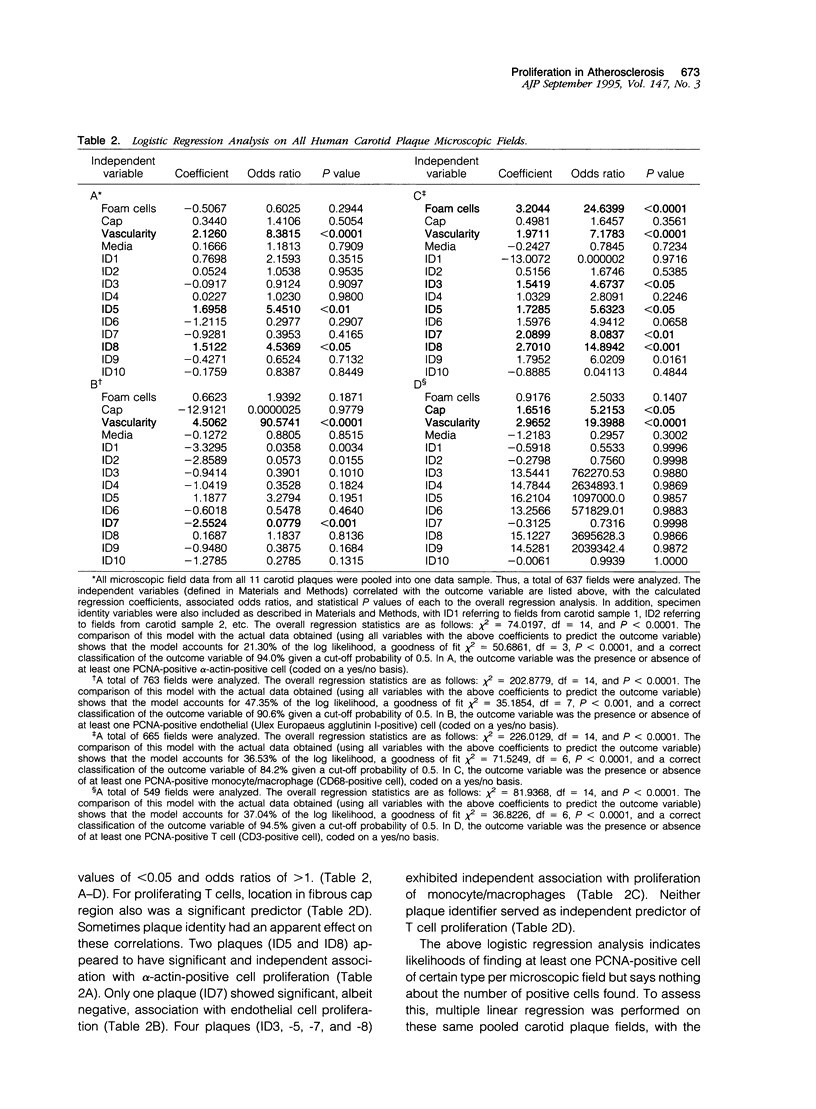
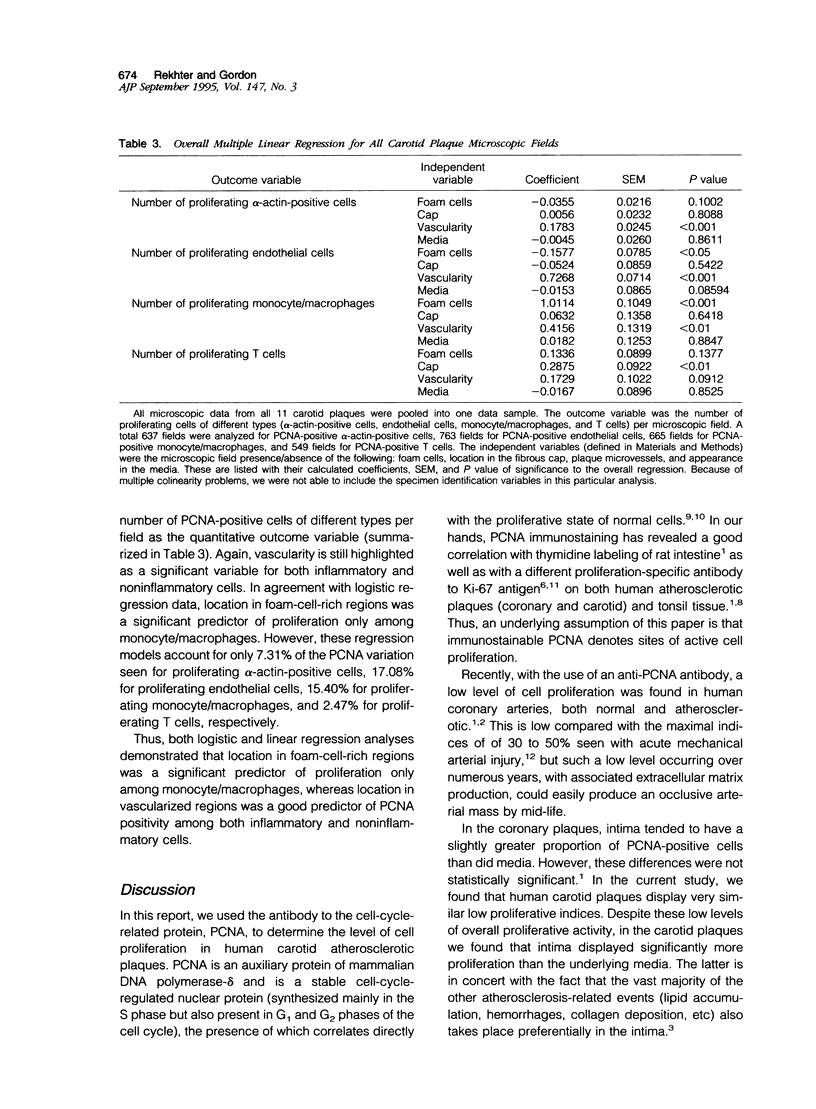



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Brogi E., Winkles J. A., Underwood R., Clinton S. K., Alberts G. F., Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993 Nov;92(5):2408–2418. doi: 10.1172/JCI116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Daoud A. S., Jones R., Scott R. F., Morrison E. S., Kim D. N., Lee K. T., Thomas W. A., Dodds W. J. Dietary-induced atherosclerosis in miniature swine. Exp Mol Pathol. 1968 Jun;8(3):263–301. doi: 10.1016/s0014-4800(68)80001-2. [DOI] [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Lee K. T., Lee K. J., Thomas W. A. Increased mitotic activity in aortas of swine after three days of cholesterol feeding. Arch Pathol. 1969 Nov;88(5):463–469. [PubMed] [Google Scholar]
- Geer J. C., McGill H. C., Jr, Robertson W. B., Strong J. P. Histologic characteristics of coronary artery fatty streaks. Lab Invest. 1968 May;18(5):565–570. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda S., Coltrera M. D., Ross R., Gown A. M. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol. 1993 Jun;142(6):1787–1793. [PMC free article] [PubMed] [Google Scholar]
- Kim D. N., Imai H., Schmee J., Lee K. T., Thomas W. A. Intimal cell mass-derived atherosclerotic lesions in the abdominal aorta of hyperlipidemic swine. Part 1. Cell of origin, cell divisions and cell losses in first 90 days on diet. Atherosclerosis. 1985 Aug;56(2):169–188. doi: 10.1016/0021-9150(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Kim D. N., Schmee J., Ho H. T., Thomas W. A. The "turning off" of excessive cell replicative activity in advanced atherosclerotic lesions of swine by a regression diet. Atherosclerosis. 1988 Jun;71(2-3):131–142. doi: 10.1016/0021-9150(88)90137-2. [DOI] [PubMed] [Google Scholar]
- Kim D. N., Schmee J., Lee K. T., Thomas W. A. Atherosclerotic lesions in the coronary arteries of hyperlipidemic swine. Part 1. Cell increases, divisions, losses and cells of origin in first 90 days on diet. Atherosclerosis. 1987 Apr;64(2-3):231–242. doi: 10.1016/0021-9150(87)90251-6. [DOI] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Liptay M. J., Parks W. C., Mecham R. P., Roby J., Kaiser L. R., Cooper J. D., Botney M. D. Neointimal macrophages colocalize with extracellular matrix gene expression in human atherosclerotic pulmonary arteries. J Clin Invest. 1993 Feb;91(2):588–594. doi: 10.1172/JCI116238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- O'Brien E. R., Garvin M. R., Dev R., Stewart D. K., Hinohara T., Simpson J. B., Schwartz S. M. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol. 1994 Oct;145(4):883–894. [PMC free article] [PubMed] [Google Scholar]
- Orekhov A. N., Kosykh V. A., Repin V. S., Smirnov V. N. Cell proliferation in normal and atherosclerotic human aorta. I. Flow cytofluorometric determination of cellular deoxyribonucleic acid content. Lab Invest. 1983 Apr;48(4):395–398. [PubMed] [Google Scholar]
- Rekhter M. D., Gordon D. Cell proliferation and collagen synthesis are two independent events in human atherosclerotic plaques. J Vasc Res. 1994 Sep-Oct;31(5):280–286. doi: 10.1159/000159054. [DOI] [PubMed] [Google Scholar]
- Rekhter M. D., Gordon D. Does platelet-derived growth factor-A chain stimulate proliferation of arterial mesenchymal cells in human atherosclerotic plaques? Circ Res. 1994 Sep;75(3):410–417. doi: 10.1161/01.res.75.3.410. [DOI] [PubMed] [Google Scholar]
- Rekhter M. D., Zhang K., Narayanan A. S., Phan S., Schork M. A., Gordon D. Type I collagen gene expression in human atherosclerosis. Localization to specific plaque regions. Am J Pathol. 1993 Dec;143(6):1634–1648. [PMC free article] [PubMed] [Google Scholar]
- Rekhter M., Nicholls S., Ferguson M., Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993 Apr;13(4):609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990 Sep-Oct;10(5):680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Scott R. F., Thomas W. A., Kim D. N., Schmee J. Endothelial cell labeling indices in swine aortas in relation to intimal cell mass-derived atherosclerotic lesions. Atherosclerosis. 1985 Sep;56(3):263–270. doi: 10.1016/0021-9150(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Solberg L. A., Strong J. P. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983 May-Jun;3(3):187–198. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- Stemme S., Hansson G. K. Immune mechanisms in atherosclerosis. Coron Artery Dis. 1994 Mar;5(3):216–222. doi: 10.1097/00019501-199403000-00006. [DOI] [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Stemme S., Rymo L., Hansson G. K. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991 Dec;65(6):654–660. [PubMed] [Google Scholar]
- Swanson S. J., Rosenzweig A., Seidman J. G., Libby P. Diversity of T-cell antigen receptor V beta gene utilization in advanced human atheroma. Arterioscler Thromb. 1994 Jul;14(7):1210–1214. doi: 10.1161/01.atv.14.7.1210. [DOI] [PubMed] [Google Scholar]
- Velican C., Velican D. Progression of coronary atherosclerosis from adolescents to mature adults. Atherosclerosis. 1983 May;47(2):131–144. doi: 10.1016/0021-9150(83)90150-8. [DOI] [PubMed] [Google Scholar]
- Walker L. N., Reidy M. A., Bowyer D. E. Morphology and cell kinetics of fatty streak lesion formation in the hypercholesterolemic rabbit. Am J Pathol. 1986 Dec;125(3):450–459. [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]